Polyatomic Ions: Comprehensive Understanding Of Composition, Charge, And Characteristics
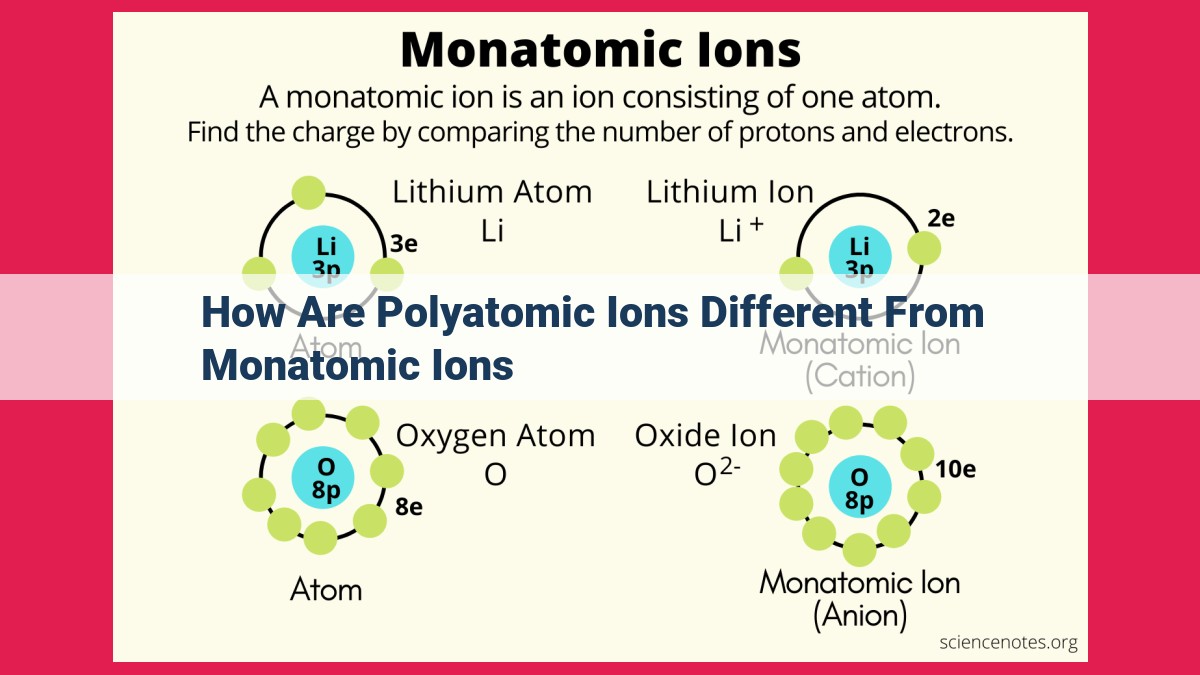
Polyatomic ions differ from monatomic ions in their composition, charge, formation, stability, and behavior. Monatomic ions consist of a single atom with a charge, while polyatomic ions are composed of groups of atoms with a net charge. Polyatomic ions have a central atom surrounded by other atoms or ions, creating a complex structure. The charge of a monatomic ion is equal to the charge of the atom, while the charge of a polyatomic ion is the sum of the charges of its constituent atoms or ions. Monatomic ions form through electron loss or gain, whereas polyatomic ions form through chemical bonding. Polyatomic ions are more stable due to charge distribution, which enhances their solubility and conductivity in solutions. Additionally, polyatomic ions have specific naming conventions that indicate the number and types of atoms present, as well as their oxidation states.
Explain the difference in composition between monatomic ions (single atoms with a charge) and polyatomic ions (groups of atoms with a charge).
How Are Polyatomic Ions Different from Monatomic Ions?
In the realm of chemistry, ions are atoms or groups of atoms that carry an electrical charge. Monatomic ions consist of single atoms that have lost or gained electrons, resulting in a positive or negative charge. In contrast, polyatomic ions are groups of atoms that are covalently bonded together and carry multiple charges. Let’s delve deeper into their differences in composition.
Composition
-
Monatomic ions: As their name suggests, monatomic ions are made up of single atoms that have gained or lost one or more electrons. Examples include sodium (Na+) and chloride (Cl-) ions.
-
Polyatomic ions: Polyatomic ions, on the other hand, are composed of two or more atoms that are covalently bonded together. They form when atoms donate or share electrons to achieve a stable electron configuration. Examples include the nitrate (NO3-) ion, which consists of a nitrogen atom bonded to three oxygen atoms, and the sulfate (SO42-) ion, which consists of a sulfur atom bonded to four oxygen atoms.
-
Central atom and surrounding atoms/ions: In polyatomic ions, there is typically a central atom, which is usually a metal or nonmetal, surrounded by other atoms or ions called ligands. The ligands can be either atoms or other polyatomic ions.
**How Polyatomic Ions Differ from Monatomic Ions: Unraveling the Chemical Intrigue**
Monatomic and polyatomic ions, electric counterparts of atoms, play pivotal roles in the realm of chemistry. While they share the characteristic charge that distinguishes them from neutral atoms, their compositions and behaviors set them apart.
Polyatomic Ions: A Symphony of Atoms
Unlike monatomic ions that consist of a solitary atom bearing a charge, polyatomic ions are captivating ensembles of atoms or ions that bond together to form a cohesive unit with a collective charge. At the heart of these structures lies a central atom, the nucleus around which other atoms or ions merrily dance, completing the molecular arrangement.
Consider the sulfate ion (SO4 2-), a ubiquitous player in chemistry. Oxygen atoms gracefully encircle a central sulfur atom, each contributing electrons to form covalent bonds. This dance creates a stable, charged entity that captivates scientists and shapes the chemical landscape.
The Charge Dance: A Balancing Act
Monatomic ions waltz with a single charge, either positive or negative, reflecting the loss or gain of electrons. Polyatomic ions, on the other hand, carry a collective charge that summates the charges of their constituent atoms or ions.
The sulfate ion, with its two negative charges, aptly illustrates this concept. Each oxygen atom contributes a -2 charge, while the central sulfur atom contributes a +6 charge. The algebraic sum (-2 x 4 + +6) yields the overall charge of -2.
Formation: A Tale of Chemical Ballet
Monatomic ions emerge from the loss or gain of electrons by atoms, a process that alters the atomic equilibrium and bestows a charge. Sodium (Na), for instance, willingly surrenders an electron, becoming a positively charged sodium ion (Na+). Chlorine (Cl) eagerly accepts an electron, transforming into a negatively charged chloride ion (Cl-).
Polyatomic ions, in contrast, owe their existence to the chemical bonding of atoms or ions. These entities may form through covalent bonds, sharing electrons to achieve stability, or through ionic bonds, where one atom donates electrons to another.
Stability: A Measure of Chemical Grace
Monatomic ions, adorned with a singular charge and simple structure, exhibit greater stability compared to their polyatomic counterparts. Their unadorned nature grants them a robust resilience that withstands the chemical rigors of their environment.
Polyatomic ions, with their intricate molecular architecture, find stabilization through the distribution of their charge. The dispersal of charge across multiple atoms reduces the electrostatic repulsion that would otherwise destabilize the ion. Thus, polyatomic ions dance gracefully in chemical solutions, their stability a testament to the harmony of their atomic ensemble.
Behavior: A Symphony of Interactions
Monatomic ions prefer solitude in aqueous solutions, mingling sparingly with other ions. Their isolated existence allows them to maintain their individuality, influencing the solution’s properties solely through their charge.
Polyatomic ions, however, engage in a dynamic interplay with neighboring ions, influencing a solution’s solubility, conductivity, and other attributes. Their collective charge and molecular complexity endow them with a charisma that shapes the chemical landscape.
Nomenclature: A Language of Ion Identity
Monatomic ions proudly bear the name of their parent element, adorned with suffixes that reveal their charge. Sodium ion (Na+) and chloride ion (Cl-) exemplify this straightforward naming convention.
Polyatomic ions don unique names that reflect their composition and oxidation states. The sulfate ion, for instance, elegantly conveys its sulfur content and -2 oxidation state. This nomenclature system empowers chemists to decipher the chemical makeup of polyatomic ions with ease.
How Polyatomic Ions Differ from Monatomic Ions: A Comprehensive Comparison
When it comes to ions, you might encounter two main types: monatomic and polyatomic. While both are charged particles, they possess distinct characteristics that set them apart. Let’s dive into their differences and explore the fascinating world of ions!
Charge: A Sum of Parts
Monatomic ions are formed when an atom gains or loses electrons, resulting in a single positive or negative charge. For instance, when sodium (Na) loses an electron, it transforms into a positively charged sodium ion (Na+). On the other hand, polyatomic ions are groups of atoms that carry a net charge. They consist of a central atom surrounded by other atoms or ions, forming a stable and cohesive unit. The overall charge of a polyatomic ion is the sum of the charges of its constituent ions. For example, the carbonate ion (CO32-) consists of a central carbon atom bonded to three oxygen atoms, resulting in a net charge of -2.
Formation: A Tale of Interactions
Monatomic ions form through simple electron transfer reactions. Atoms lose or gain electrons to achieve a stable electron configuration. In contrast, polyatomic ions are created through chemical bonding between atoms or ions. They exist as stable entities with strong electrostatic forces holding them together. These bonds can be covalent, ionic, or a combination of both. For instance, the sulfate ion (SO42-) forms when a sulfur atom bonds with four oxygen atoms, resulting in a tetrahedral structure with a net charge of -2.
Stability: The Power of Charge Distribution
Monatomic ions tend to be relatively stable due to their simple structure and single charge. However, as we move to polyatomic ions, their increased complexity introduces a unique advantage: enhanced stability. The charge in polyatomic ions is distributed over multiple atoms, reducing the charge density and increasing their resistance to dissociation. This distributed charge also makes polyatomic ions less reactive than monatomic ions in certain chemical reactions.
How Are Polyatomic Ions Different from Monatomic Ions?
Ever wonder why some ions travel solo, while others form cozy groups? In the world of ions, we have two distinct types: monatomic ions and polyatomic ions. They may share the same electrical charge, but their compositions, behaviors, and personalities are as different as night and day.
Composition: The Lone Wolf vs. The United Front
Monatomic ions are like loners. They consist of a single atom that has lost or gained one or more electrons, leaving it with a positive or negative charge. Polyatomic ions, on the other hand, are like families or teams. They’re made up of multiple atoms that are chemically bonded together, forming a stable unit with a net charge.
Charge: Adding Up the Electrical Score
Both monatomic and polyatomic ions are charged, but their charges are determined differently. Monatomic ions have a simple charge, either positive (for metal ions) or negative (for nonmetal ions). Polyatomic ions, however, have a composite charge that is the sum of the charges on the individual atoms or ions making them up. This net charge is what determines their overall behavior in chemical reactions.
Formation: A Tale of Loneliness and Teamwork
Monatomic ions form when atoms undergo a simple process called electron transfer. An atom donates or receives electrons, resulting in a net gain or loss of charge. Polyatomic ions, however, require a bit more cooperation. They form when atoms share electrons or transfer electrons to each other, creating a stable chemical bond that holds the ions together.
Stability: The Power of Distribution
Monatomic ions are relatively stable due to their simple structure. However, polyatomic ions take stability to a whole new level. Their charge is distributed over multiple atoms, which makes them less reactive and more resistant to breaking apart. This enhanced stability gives them a unique advantage in chemical processes.
Behavior: Solo Acts vs. Team Players
In solution, monatomic ions act like individuals. They exist independently, each ion having its own unique properties. Polyatomic ions, on the other hand, behave like teams. They interact with each other, influencing their solubility, conductivity, and other chemical properties. Their presence can significantly alter the behavior of solutions compared to monatomic ions alone.
Nomenclature: Naming the Lone Rangers and the Team Players
Monatomic ions follow a straightforward naming convention. They’re named based on the element name and the suffix “-ide” (for negative ions) or “-ium” (for positive ions). Polyatomic ions, however, have specific names that indicate the number and types of atoms present, as well as their oxidation states. These names are essential for identifying and understanding their complex chemical behaviors.
How Are Polyatomic Ions Different from Monatomic Ions?
In the vast realm of chemistry, ions play a crucial role in shaping the properties of matter. Among these ions, two distinct types stand out: monatomic and polyatomic ions. While they share the common characteristic of carrying an electrical charge, their compositions and behaviors set them apart.
Monatomic Ions: The Lone Warriors
Imagine an atom, a tiny building block of matter. Its core, known as the nucleus, houses positively charged protons and uncharged neutrons. Orbiting around the nucleus are negatively charged electrons. When an atom loses or gains electrons, it transforms into an ion. A monatomic ion is an ion that consists of a single atom with a charge.
The formation of monatomic ions is a straightforward process. When an atom loses an electron, it becomes a positively charged ion known as a cation. Conversely, when an atom gains an electron, it transforms into a negatively charged ion called an anion. The charge of a monatomic ion is determined by the number of electrons lost or gained. For instance, sodium (Na) can lose one electron to form a cation with a charge of +1, written as Na+.
Polyatomic Ions: The Cooperative Groups
In contrast to monatomic ions, polyatomic ions are composed of multiple atoms that form a charged unit. These ions are like chemical teams, where each atom contributes its electrons to the overall charge. The central atom, typically a nonmetal, attracts surrounding atoms or ions, creating a stable arrangement.
Take the example of the nitrate ion, NO₃⁻. It consists of one nitrogen atom (N) bonded to three oxygen atoms (O). The nitrogen atom has a +5 oxidation state, while each oxygen atom carries a -2 oxidation state. This results in an overall charge of -1 for the nitrate ion.
The formation of polyatomic ions involves chemical bonding between the constituent atoms. The specific type of bond will depend on the nature of the atoms involved. For instance, the nitrate ion is formed through covalent bonding, where the atoms share electrons to create a stable structure.
Stability and Behavior: A Tale of Two Ions
Monatomic ions, with their simple structure, exhibit relative stability. They behave as independent entities in solution, existing as isolated ions. Polyatomic ions, on the other hand, gain enhanced stability due to the distribution of charge among multiple atoms. This charge distribution helps to stabilize the ion and prevent it from breaking apart.
The behavior of polyatomic ions in solution is influenced by their interactions with other molecules. They can form hydrogen bonds with water molecules, affecting their solubility and conductivity. Additionally, their overall charge can impact the properties of the solution, such as its pH or freezing point.
How Are Polyatomic Ions Different from Monatomic Ions?
When atoms undergo chemical reactions, they can gain or lose electrons, transforming into ions. These ions can be monatomic, consisting of a single atom with a charge, or polyatomic, made up of a group of atoms with a collective charge. While both types of ions share similar properties, there are key differences that set them apart.
Composition
- Monatomic ions are single atoms that have gained or lost electrons, resulting in an overall charge.
- Polyatomic ions are groups of atoms that carry a charge as a unit. They typically consist of a central atom and one or more surrounding atoms or ions.
Charge
- Monatomic ions have a single charge, which is determined by the number of electrons gained or lost.
- Polyatomic ions have a charge that is the sum of the charges of the individual atoms or ions they contain. This net charge is often indicated by a superscript, such as sulfate (SO4)2-.
Formation
- Monatomic ions are formed when atoms lose or gain electrons to achieve a stable electron configuration. This can occur through chemical reactions or physical processes like ionization.
- Polyatomic ions are formed through chemical bonding, where atoms or ions come together to share electrons. This often involves the formation of covalent or ionic bonds, creating a stable molecular structure.
Stability
- Monatomic ions are generally more stable than polyatomic ions due to their simpler structure. They have a single atom with a well-defined electron configuration.
- Polyatomic ions can be more stable than monatomic ions when their charge is distributed over multiple atoms, reducing the electrostatic repulsion between electrons.
Behavior
- Monatomic ions tend to exist independently in solution, primarily interacting with other ions through electrostatic forces.
- Polyatomic ions can interact with other ions or molecules due to their complex structure and charge distribution. This can influence their solubility, conductivity, and other properties in solution.
Nomenclature
- Monatomic ions are named using the element name and a suffix (-ide for anions, -ium for cations).
- Polyatomic ions have specific names that indicate the number and types of atoms present, as well as their oxidation states. These names often follow a systematic convention based on their chemical composition.
How Are Polyatomic Ions Different from Monatomic Ions?
In the fascinating realm of chemistry, ions play a crucial role, with monatomic ions and polyatomic ions taking center stage. Join us as we delve into their unique characteristics and explore the fundamental differences that set them apart.
1. Composition: A Defining Distinction
Monatomic ions are solitary entities, consisting of a single atom that has either gained or lost electrons, leaving them with an electrical charge. In contrast, polyatomic ions are chemical groups that pack a punch, comprising multiple atoms held together by covalent bonds. They behave as a single unit, carrying an overall charge.
2. Charge: A Matter of Magnitude
Monatomic ions carry a single charge, reflecting their simple atomic structure. Polyatomic ions, however, exhibit more complex behavior. Their charge results from the sum of the charges carried by their constituent atoms, leading to a net charge that can vary depending on the specific combination of elements.
3. Formation: A Tale of Bonding
Monatomic ions arise when atoms undergo electron transfer, either losing or gaining electrons to achieve a stable electronic configuration. Ionic bonds form between these charged species, creating compounds. Polyatomic ions, on the other hand, result from covalent bonding between atoms within a molecule. These bonds share electrons, resulting in stable molecular structures.
4. Stability: Strength in Simplicity vs. Charge Distribution
Monatomic ions enjoy a level of stability due to their simple structure and absence of internal bonding. Polyatomic ions, with their more complex arrangements, gain enhanced stability from the distribution of charge across multiple atoms. This charge dispersal strengthens their internal bonds and increases their overall stability.
Enhanced Stability of Polyatomic Ions: A Tale of Charge Distribution
In the world of chemistry, stability is paramount. It’s the holy grail that every ion aspires to. Monatomic ions, those solitary atoms that carry a charge, enjoy a certain level of stability due to their simplicity. But polyatomic ions – the powerhouses of the ion kingdom – take stability to a whole new level.
The secret to their extraordinary resilience lies in their unique charge distribution. Unlike monatomic ions, which concentrate their charge on a single atom, polyatomic ions spread their charge among several atoms. Think of it as a team effort, where the burden of charge is shared by all.
This charge distribution has profound implications for the stability of polyatomic ions. Imagine a basketball being held by a single person. The weight of the ball presses down on the person’s hands, potentially causing discomfort or even injury. Now imagine that weight being distributed among a team of ten people. The burden on each individual is significantly reduced, resulting in greater stability and ease.
Similarly, in polyatomic ions, the charge distribution reduces the stress on any single atom. The charge is more evenly dispersed, minimizing the risk of instability or breakdown. This collective responsibility strengthens the polyatomic ion as a whole, making it less susceptible to disruptive forces.
So, there you have it: the secret behind the enhanced stability of polyatomic ions. It’s all about charge distribution, the team effort that keeps these molecular marvels strong and resilient.
How Polyatomic Ions Differ from Monatomic Ions: A Tale of Composition, Charge, and More
In the vast realm of chemistry, ions reign supreme as electrically charged entities. These charged particles play a pivotal role in shaping the properties of matter, governing everything from chemical reactions to the flow of electricity. Among these ions, monatomic ions and polyatomic ions stand out as two distinct yet fascinating classes.
Composition: A Matter of Singularity and Collectivity
Monatomic ions, as their name suggests, are singular atoms that have either gained or lost electrons, acquiring an overall charge. Sodium ions (Na+) and chloride ions (Cl-) are classic examples of monatomic ions, embodying the simplicity of a single atomic entity.
Polyatomic ions, on the other hand, are complexes of multiple atoms that collectively bear a charge. They resemble molecular entities, with atoms bonded together via covalent or ionic bonds. Examples include the nitrate ion (NO3-), which comprises one nitrogen atom and three oxygen atoms, and the ammonium ion (NH4+), which consists of one nitrogen atom and four hydrogen atoms.
Charge: A Reflection of Composition
The charge of an ion is a fundamental property that governs its behavior in chemical reactions. Monatomic ions carry a single charge, which is equal to the number of electrons gained or lost by the atom. Sodium ions, having lost one electron, carry a +1 charge, while chloride ions, having gained one electron, carry a -1 charge.
Polyatomic ions, in contrast, exhibit a charge that is the sum of the charges of their constituent atoms or ions. The nitrate ion, for instance, has a charge of -1, reflecting the combined charges of the nitrogen atom (+5) and the three oxygen atoms (-2 each). The ammonium ion, on the other hand, carries a +1 charge, resulting from the combination of the nitrogen atom (-3) and the four hydrogen atoms (+1 each).
Behavior in Solution: A Manifestation of Complexity
In solution, monatomic ions behave as isolated entities, exhibiting a high degree of mobility and independence. They can freely move and interact with other ions in the solution, forming electrostatic interactions that govern their behavior.
Polyatomic ions, however, display a more complex behavior in solution. Their larger size and multifaceted charge distribution influence their solubility, conductivity, and other properties. They can interact with each other and with solvent molecules, forming intricate networks that affect the overall behavior of the solution.
In summary, monatomic ions and polyatomic ions represent two distinct classes of ions with unique compositions, charges, and behaviors. Understanding these differences is essential for comprehending the intricate world of chemistry and the diverse roles that ions play in shaping the properties of matter.
How Polyatomic Ions Differ from Monatomic Ions: Unraveling Their Unique Interactions
In the fascinating world of chemistry, ions play a crucial role, influencing a multitude of chemical processes and properties. Among these ions, monatomic ions and polyatomic ions stand apart, each possessing distinctive characteristics. While monatomic ions consist of a lone atom bearing a charge, polyatomic ions are intriguing assemblies of multiple atoms bonded together to form a charged species.
Delving into the Interactions of Polyatomic Ions
The complex nature of polyatomic ions gives rise to unique interactions that significantly impact their behavior in chemical systems. These interactions extend beyond their charge and encompass their solubility, conductivity, and other physiochemical properties.
Solubility: A Tale of Attraction and Repulsion
Polyatomic ions interact differently with water molecules than monatomic ions. The charge distribution within polyatomic ions creates regions of both positive and negative charge, leading to complex interactions with water molecules. These electrostatic forces can either enhance or diminish the solubility of polyatomic ions in aqueous solutions.
Conductivity: A Symphony of Ion Mobility
The charged nature of polyatomic ions grants them the ability to conduct electricity. The mobility of these ions in solution depends on their size, shape, and charge distribution. Polyatomic ions, with their intricate structures, often have lower mobility than monatomic ions, which have a more straightforward spherical shape and single charge.
Other Properties: Uncovering Hidden Influences
Beyond solubility and conductivity, polyatomic ions also influence other properties of chemical systems. Their complex structures can affect their reactivity, stability, and even color. These subtle yet significant effects highlight the multifaceted nature of polyatomic ions and their profound impact on chemical processes.
In summary, polyatomic ions, with their intricate composition and unique interactions, play a vital role in shaping the behavior of chemical systems. Their influence on solubility, conductivity, and other properties underscores their importance in understanding the intricacies of chemical reactions and their applications in various fields.
How Are Polyatomic Ions Different from Monatomic Ions?
Unveiling the fascinating world of chemistry, we explore the intriguing differences between monatomic and polyatomic ions. Embark on a storytelling journey as we unravel their compositions, charges, and behaviors.
Composition: A Tale of One vs. Many
Monatomic ions, like intrepid warriors, consist of single atoms that have lost or gained electrons. Polyatomic ions, in contrast, are complex groups of atoms that share a charge. They possess a central atom, flanked by loyal attendants of surrounding atoms or ions.
Charge: A Balancing Act
Monatomic ions carry a single charge, either positive or negative. Polyatomic ions, however, have a charge that is the sum of the charges of their constituent ions. This intricate dance of charges results in a delicate net charge that defines their unique properties.
Formation: From Loss to Bonds
Monatomic ions are formed when atoms shed or embrace electrons, becoming electrically charged species. Polyatomic ions, on the other hand, arise from the chemical bonding of atoms or ions. These intricate bonds create strong bonds that hold the ions together.
Stability: Strength in Simplicity and Numbers
Monatomic ions, with their simple structure, exhibit relative stability. Polyatomic ions, however, shine with enhanced stability due to the distribution of their charge across multiple atoms. This collaborative approach strengthens their bonds and boosts their resilience.
Behavior: Interplay in Solutions
Monatomic ions, akin to solitary wanderers, behave independently in solution. Polyatomic ions, however, are social butterflies that interact with each other, influencing properties like solubility and conductivity. Their captivating interactions paint a complex tapestry of chemical behaviors.
Nomenclature: Naming the Ions
Monatomic ions follow a straightforward naming convention, incorporating the element name with suffixes like “-ide” or “-ium”. Polyatomic ions, however, boast specific names that reveal their composition and oxidation states. These names provide valuable insights into their unique chemical identities.
Polyatomic Ions: Unraveling the Intriguing Differences from Monatomic Ions
While both monatomic and polyatomic ions possess a magnetic charge, they exhibit striking differences in their composition and behavior. Polyatomic ions steal the spotlight with their complex structure, which comprises a central atom surrounded by a captivating entourage of other atoms. This entourage, like a well-orchestrated dance, determines the ion’s charge. Unlike monatomic ions with their solitary charge, polyatomic ions showcase a net charge, the result of balancing the dance between the different charges of their constituent atoms.
The formation of these intricate polyatomic ions is a tale of chemical bonding, where atoms and ions join hands to create a stable molecular ecosystem. The stability of these ions is a marvel in itself. While monatomic ions stand alone, unperturbed in their simplicity, polyatomic ions find harmony in their distributed charge, granting them enhanced stability.
The presence of polyatomic ions in a solution is like a social gathering, influencing the solubility and conductivity of the liquid. They form a vibrant network of interactions, unlike monatomic ions, which prefer a more isolated existence.
To unravel the secrets of these polyatomic ions, scientists have developed a precise naming convention. Each ion bears a unique title, reflecting the number and types of atoms present within its structure. These names reveal the oxidation states of the constituent atoms, providing a glimpse into the ion’s intricate chemistry.
As you delve into the world of polyatomic ions, remember that their complexities add a dash of intrigue to the study of ions. Their unique structure and behavior make them an essential part of the chemical landscape, waiting to be discovered.