Platinum: Unveiling The Significance Of Neutron Count (117 Neutrons)

Platinum, a valuable metal, has
- 117 neutrons
in its most common isotope. Understanding the number of neutrons is crucial for determining atomic mass, predicting nuclear stability, and exploring isotope variations that influence elemental properties and applications.
- Describe the importance of platinum in various applications.
- State the goal of the article to determine the number of neutrons in platinum.
Unlocking the Secrets of Platinum: Unraveling its Neutron Count
Platinum, a precious metal renowned for its luster and versatility, holds immense significance across diverse industries. Its exceptional durability, resistance to corrosion, and catalytic properties make it indispensable in applications ranging from jewelry and dentistry to automotive catalytic converters and electronics. Understanding the number of neutrons within platinum’s atomic structure is crucial for comprehending its behavior and optimizing its utilization.
This exploration delves into the fascinating realm of nuclear physics to determine the number of neutrons in platinum. By unraveling the mysteries surrounding mass number, atomic mass, isotopes, and neutron-to-proton ratio, we gain a deeper appreciation for the intricate workings of this enigmatic element.
Understanding Mass Number and Atomic Mass: The Building Blocks of Platinum
In our exploration to unravel the secrets of platinum’s atomic structure, we delve into the fundamental concepts of mass number and atomic mass. Understanding these concepts is crucial to determining the number of neutrons in this precious metal.
The Concept of Mass Number
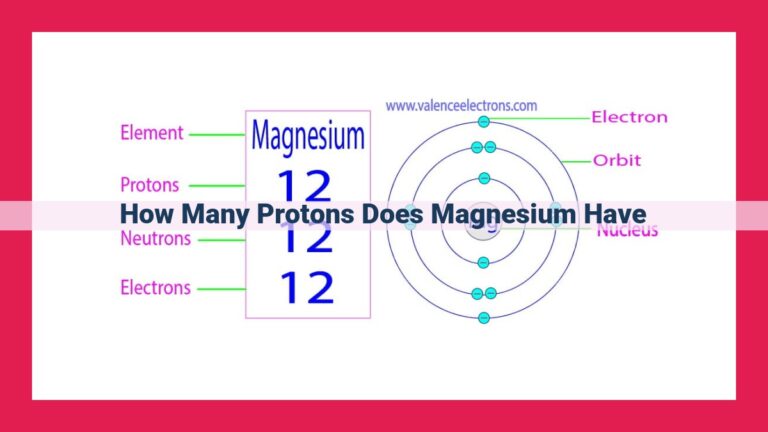
At the heart of an atom lies its nucleus, a bustling hub of protons and neutrons. The mass number of an atom, denoted by the symbol A, represents the total number of these subatomic particles residing within the nucleus. It is calculated as the sum of the number of protons (Z) and the number of neutrons (N):
A = Z + N
The Relationship between Atomic Mass and Neutrons
The atomic mass of an element, expressed in atomic mass units (amu), represents the average mass of its naturally occurring isotopes. Isotopes are atoms of the same element with different neutron counts, and they contribute to the overall atomic mass in proportion to their abundance.
The number of neutrons significantly influences the atomic mass. Adding neutrons to the nucleus increases the overall mass without affecting the number of protons. As a result, isotopes with more neutrons will have higher atomic masses. Conversely, isotopes with fewer neutrons will have lower atomic masses.
By comprehending these concepts, we lay the groundwork for determining the number of neutrons in platinum and unlocking the mysteries of its atomic structure.
Exploring Isotopes and Their Role
In the vast expanse of the atomic realm, where the fundamental building blocks of matter reside, isotopes play a pivotal role in shaping the behavior and characteristics of elements. Isotopes are atoms of the same element that share an identical number of protons but differ in the number of neutrons within their nuclei.
Imagine a bustling city where each atom represents a unique building. Protons, like the steadfast pillars of these buildings, determine the element’s identity. Neutrons, on the other hand, act as the flexible floors, varying in number while maintaining the overall structure. This subtle variation in neutron count gives rise to the fascinating diversity of isotopes.
For example, the element platinum, renowned for its catalytic prowess and shimmering brilliance, has six naturally occurring isotopes. They share the same 78 protons, but their neutron counts range from 114 to 119. These isotopes, with their distinct neutron populations, collectively determine platinum’s average atomic mass, making it heavier than gold despite having fewer protons.
The abundance of different isotopes within an element plays a crucial role in its atomic mass. Isotopes with higher neutron numbers are generally less abundant than those with lower neutron counts. This variation in abundance influences the overall weight of the element. In the case of platinum, the most abundant isotope has 117 neutrons, accounting for 63% of its atomic mass.
Understanding isotopes and their impact on atomic mass is fundamental to comprehending the diversity of matter. It provides a framework for unraveling the properties and behavior of elements, paving the way for advancements in scientific research and technological innovations.
Examining Neutron-to-Proton Ratio: The Key to Nuclear Stability
Unveiling the intricate world of elements, we embark on a journey to unravel a fundamental aspect of their atomic composition: the neutron-to-proton ratio. This enigmatic relationship holds the secret to nuclear stability, preventing elements from succumbing to radioactive decay.
The atomic nucleus, the heart of an atom, is composed of protons and neutrons. While protons are positively charged, neutrons are neutral in charge. The number of protons determines the element’s identity, while the number of neutrons influences its stability.
A delicate balance exists between protons and neutrons. Too many protons relative to neutrons can render the nucleus unstable, causing it to emit particles in a process known as radioactive decay. Conversely, a surplus of neutrons can result in the nucleus absorbing protons, leading to radioactive decay.
The optimal neutron-to-proton ratio ensures nuclear stability. For lighter elements, a balanced ratio is typically 1:1. As elements become heavier, the ratio shifts in favor of neutrons to maintain stability. This is because the strong nuclear force, which binds protons and neutrons together, becomes weaker as the number of protons increases.
The neutron-to-proton ratio provides insights into the behavior of elements. Elements with a low ratio, such as hydrogen and helium, are highly stable. Elements with a high ratio, such as uranium and plutonium, are prone to radioactive decay.
This delicate balance influences various nuclear processes, including fission and fusion. In nuclear fission, the nucleus of a heavy element splits into two smaller nuclei, releasing energy. This process is crucial in nuclear power plants. In nuclear fusion, two light nuclei combine to form a heavier nucleus, again releasing energy. This process powers the Sun and other stars.
In conclusion, the neutron-to-proton ratio is a fundamental aspect of nuclear structure. It ensures stability by preventing radioactive decay and influences nuclear processes such as fission and fusion. Understanding this concept is crucial for unraveling the behavior of elements and unlocking the secrets of the atomic world.
Insights into Nuclear Structure
At the heart of every atom lies a dense core known as the nucleus. Within this tiny realm, protons, positively charged particles, and neutrons, their neutral counterparts, coexist in a delicate balance. The number of protons determines an element’s identity, while the number of neutrons significantly influences its properties.
The strong nuclear force, a powerful attraction between protons and neutrons, binds these particles together. This force overcomes the repulsive electrostatic force between positively charged protons, ensuring nuclear stability. The number of neutrons in a nucleus plays a crucial role in determining its stability.
A stable nucleus maintains a relatively balanced ratio of neutrons to protons. This ratio, known as the neutron-to-proton ratio, influences the nucleus’s ability to resist radioactive decay. When the ratio falls outside a certain range, the nucleus becomes unstable and undergoes radioactive transformations to achieve stability.
The number of neutrons also affects nuclear properties such as mass, spin, and magnetic moment. Heavier elements tend to have a higher proportion of neutrons, which contributes to their greater mass. Spin, a fundamental property of elementary particles, is influenced by the number of neutrons and protons in the nucleus. Similarly, the magnetic moment, which arises from the nucleus’s intrinsic magnetic field, is affected by the arrangement of protons and neutrons.
Understanding the intricate dynamics of nuclear structure is essential for comprehending the behavior and properties of elements. It provides insights into the stability, reactivity, and applications of various materials, from nuclear energy to medical diagnostics. By delving into the realm of nuclear structure, scientists unlock the secrets of the atomic world, paving the way for technological advancements and a deeper understanding of the universe.
Exploring Radioactivity and Its Implications
In the realm of nuclear physics, radioactivity unveils a captivating world of atomic transformations. Radioactivity is the inherent property of unstable atomic nuclei to emit energy and particles in an attempt to achieve a more stable configuration. This process, known as radioactive decay, involves the emission of different types of radiation, such as alpha particles, beta particles, and gamma rays.
A crucial factor influencing nuclear stability is the neutron-to-proton ratio. In stable nuclei, the number of neutrons and protons is balanced, providing a stable equilibrium. However, when the neutron-to-proton ratio becomes imbalanced, the nucleus becomes unstable and undergoes radioactive decay to restore its stability.
Radioactivity plays a pivotal role in determining the number of neutrons in an atomic nucleus. By studying the types and energy levels of emitted radiation, scientists can gain valuable insights into the nuclear composition of elements. This technique forms the foundation for determining the number of neutrons present in platinum.
For instance, one prevalent isotope of platinum, platinum-195, undergoes beta decay, where a neutron transforms into a proton and an electron, which is subsequently emitted. By measuring the energy and characteristics of the emitted beta particles, researchers can infer the change in nuclear structure and determine the number of neutrons in platinum-195.
Understanding radioactivity and its implications is essential for comprehending the fundamental properties of elements and their behavior in nuclear reactions. These concepts find practical applications in various scientific and technological fields, including nuclear medicine, radiation therapy, and radioisotope tracing techniques.