Photosynthesis: Unlocking Nature’s Energy And Life Cycle
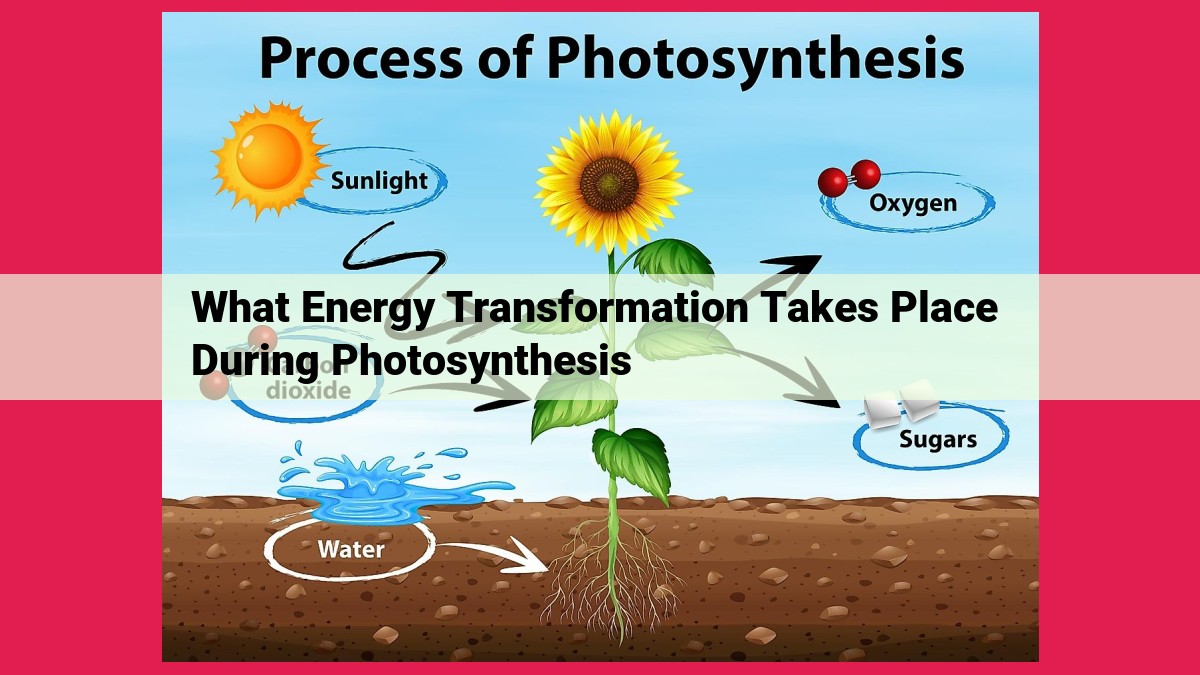
During photosynthesis, light energy is absorbed by chlorophyll molecules in plants and converted into chemical energy. This energy is then used to combine carbon dioxide and water to form glucose, a type of sugar. The energy stored in glucose is later released when it is broken down during cellular respiration. As a byproduct of photosynthesis, oxygen is released into the atmosphere. This process is crucial as it not only provides food for plants but also releases oxygen essential for life on Earth.
Photosynthesis: The Life-Giving Process That Sustains Our Planet
In the tapestry of life on Earth, photosynthesis stands as a cornerstone process, providing the very foundation for our existence. It is a remarkable dance between sunlight, water, and carbon dioxide, resulting in the creation of sustenance and the release of oxygen.
Without photosynthesis, life as we know it would cease. It is responsible for producing the food that nourishes us, from the humble blades of grass to the towering trees. The oxygen we breathe, essential for our survival, is a byproduct of this wondrous process.
The Symphony of Energy Transformation
At the heart of photosynthesis lies a symphony of energy transformation. Sunlight, a powerful form of electromagnetic energy, is captured by specialized molecules in plant cells called chlorophyll. Chlorophyll is the conductor of this symphony, converting light energy into chemical energy.
The chemical energy is then used to split water molecules into hydrogen and oxygen. The hydrogen is combined with carbon dioxide to form glucose, a simple sugar that serves as the building block for all life. The oxygen released as a byproduct is what we breathe, sustaining our very breath.
A Vital Process for a Thriving Planet
The importance of photosynthesis extends far beyond the simple provision of food and oxygen. It plays a pivotal role in regulating Earth’s atmosphere and climate. The oxygen released by photosynthesis absorbs harmful ultraviolet radiation from the sun, protecting life from its damaging effects.
Moreover, plants absorb carbon dioxide from the atmosphere, reducing greenhouse gas concentrations. Thus, photosynthesis helps mitigate climate change and ensure a stable and habitable environment for generations to come.
In conclusion, photosynthesis is the lifeblood of our planet. It nourishes us, sustains our breath, and safeguards our well-being. Its significance cannot be overstated, and its continued operation is paramount for the prosperity of life on Earth.
Types of Energy Involved in Photosynthesis: Light and Chemical Energy
Photosynthesis, the magical process through which plants weave sunlight into the fabric of life, is a testament to nature’s mastery over energy. Two distinct forms of energy take center stage in this dance of creation: light energy and chemical energy.
Light energy, the radiant messenger of the sun, embarks on a journey through the plant’s receiving antennae: pigments such as chlorophyll. These pigments, like thirsty sponges, eagerly absorb these photons, their energy eagerly welcomed into the cellular realm.
Captured light energy, like a spark, ignites the flame of chemical energy, the driving force behind photosynthesis. This energy is harnessed through intricate chemical reactions that weave together molecules like a symphony. The most significant of these is the conversion of carbon dioxide and water into glucose, a primary fuel for life.
In this transformative process, light energy propels the release of electrons from water molecules, initiating a chain reaction that generates oxygen as a byproduct. The released oxygen, a vital breath for all aerobic organisms, is expelled into the atmosphere.
Photosynthesis, a symphony of energy, weaves together light and chemical energy to sustain life on Earth. Through this intricate dance, plants nourish themselves and the planet, providing nourishment and the very air we breathe.
Energy Transformation in Photosynthesis: A Journey from Light to Life
Photosynthesis, the cornerstone of life on Earth, is an intricate process that transforms light energy into chemical energy. This energy transformation fuels the creation of food for plants and releases oxygen, the very air we breathe.
Step 1: Absorption of Light
The journey begins when chloroplasts, the energy factories within plant cells, absorb light energy from the sun. This light energy is captured by specialized pigments called chlorophyll.
Step 2: Light-Dependent Reactions
Within the thylakoid membranes of chloroplasts, light energy is used to split water molecules into hydrogen ions, oxygen, and electrons. Oxygen diffuses out of the plant, while hydrogen ions and electrons are used in the next step.
Step 3: Carbon Dioxide Fixation
In the stroma of the chloroplast, hydrogen ions, electrons, and carbon dioxide from the atmosphere are combined to form glucose, a simple sugar. This process, known as the Calvin cycle, requires the energy stored in ATP and NADPH molecules, which are produced during the light-dependent reactions.
Step 4: Release of Oxygen
As a byproduct of the light-dependent reactions, oxygen is released into the atmosphere. This oxygen is essential for respiration in plants, animals, and all other aerobic organisms.
Through this remarkable energy transformation, photosynthesis serves as the foundation of life on our planet, providing food for all living beings and replenishing the oxygen we rely on to survive.
Related Concepts: Light Energy
Photosynthesis, the life-giving process that fuels our planet, relies heavily on one crucial element: light energy. This extraordinary form of energy, a part of the vast electromagnetic spectrum, possesses a unique nature that plays an indispensable role in the transformation of sunlight into chemical energy.
At the heart of light energy lie photons, tiny bundles of electromagnetic energy, each carrying a specific amount of energy. These photons, emitted by stars like our sun, embark on a journey through space, carrying their energetic payload. When these photons encounter a plant’s pigments, they initiate a cascade of events that transforms light energy into chemical energy.
Among these pigments is chlorophyll, the primary light-absorbing molecule in plants. Chlorophyll molecules, adorned in verdant hues, act as energy transformers, converting light energy into chemical energy. This energy is then used to power the photosynthetic reactions that create food and oxygen, the very foundation of life on Earth.
The Role of Solar Energy
The sun, a celestial powerhouse, stands as the primary source of light energy for photosynthesis. Its radiant energy, emitted in the form of solar energy, travels through the vast expanse of space, carrying an immense supply of energy. When this solar energy interacts with plants, it triggers the photosynthetic machinery, setting in motion the transformation of sunlight into chemical energy.
Importance in Photosynthesis
Light energy serves as the driving force behind photosynthesis. Without this radiant energy, the conversion of carbon dioxide and water into glucose and oxygen would be impossible. Plants, the primary producers in our ecosystem, rely entirely on light energy to fuel their growth and provide sustenance for the entire food chain.
Light energy, with its unique properties and transformative power, plays a pivotal role in photosynthesis. It is the catalyst that sets in motion the chemical reactions that nourish our planet, providing the food and oxygen that sustain life. From the sun’s radiant energy to the pigments within plants, light energy is an indispensable element in the symphony of photosynthesis.
Related Concepts: Chemical Energy
Journey into the Realm of Chemical Energy
At the heart of every living organism and countless industrial processes, chemical energy reigns supreme. It’s the lifeblood that fuels our bodies and powers our world. Understanding this fundamental concept is crucial for grasping the intricate workings of photosynthesis and beyond.
Chemical Energy: Unlocking the Secrets of Matter
Chemical energy is a form of potential energy stored within the bonds that connect atoms together. When these bonds break, energy is released. Conversely, forming bonds requires an input of energy. This delicate balance between bond breakage and formation underpins chemical reactions.
The Dance of Atoms and Molecules
Atoms, the building blocks of matter, possess their own unique chemical properties. When atoms combine, they form molecules with distinct characteristics. The nature of these bonds determines the amount of chemical energy stored within the molecule.
Fuels: Harnessing the Power of Chemical Energy
In our everyday lives, we often encounter fuels such as gasoline, natural gas, and coal. These fuels contain large amounts of chemical energy, which is released when they are burned. This exothermic reaction provides heat and energy for our homes, vehicles, and industries.
Importance in Photosynthesis
In the context of photosynthesis, chemical energy plays a crucial role. During the light-dependent reactions, energy from sunlight is captured and used to break apart water molecules. This process releases oxygen as a byproduct and generates chemical energy that is stored in the form of ATP (adenosine triphosphate) and NADPH (nicotinamide adenine dinucleotide phosphate).
Chemical energy is an essential concept that pervades our world. From the food we eat to the fuel that powers our vehicles, chemical energy is the underlying force that drives countless processes. Understanding this concept is not only intellectually stimulating but also practical, as it helps us to make informed decisions about energy use and environmental sustainability.
Glucose: The Life-Sustaining Energy Source
Glucose, the foundation of energy for living organisms, is an essential molecule that plays a vital role in cellular respiration. It is a simple sugar, a type of carbohydrate, composed of carbon, hydrogen, and oxygen atoms arranged in a specific molecular structure. Glucose is the body’s primary energy source, and its availability is crucial for various cellular processes, including muscle contraction, nerve function, and the synthesis of essential biomolecules.
Plants produce glucose through the process of photosynthesis, using sunlight, carbon dioxide, and water as raw materials. This glucose is then utilized by plants for their own cellular needs or stored as starch for later use. Animals, including humans, obtain glucose from their diet, primarily from plant-based foods such as fruits, vegetables, and grains.
Once ingested, glucose is broken down through a series of metabolic pathways to release energy, which is stored in the form of adenosine triphosphate (ATP). ATP serves as the cellular energy currency, fueling all energy-requiring processes within the body. Glucose metabolism also yields carbon dioxide and water as byproducts, which are released as waste products.
The availability of glucose in the bloodstream is tightly regulated by hormones, particularly insulin and glucagon. Insulin, produced by the pancreas, promotes glucose uptake by cells and its conversion to glycogen (a storage form of glucose) in the liver and muscles. Glucagon, also produced by the pancreas, has the opposite effect, stimulating the breakdown of glycogen to release glucose into the bloodstream when needed.
Maintaining balanced glucose levels is essential for overall health. When glucose levels become too low (hypoglycemia), it can lead to symptoms such as weakness, dizziness, and even seizures. Conversely, chronically elevated glucose levels (hyperglycemia) can damage blood vessels and organs over time, increasing the risk of cardiovascular disease, kidney disease, and other health complications.
Adequate intake of glucose is crucial for optimal physical and mental performance. A diet rich in whole grains, fruits, and vegetables provides a steady supply of glucose to the body, supporting sustained energy levels and overall well-being.
Oxygen: The Vital Gas for Life and Combustion
Oxygen is an indispensable element that sustains life on Earth and fuels many essential processes. Its significance extends beyond respiration, reaching into the realms of combustion and environmental balance.
Residing in the Atmosphere and in Water Bodies
Oxygen constitutes approximately 21% of the Earth’s atmosphere, a crucial component that allows for the survival of aerobic organisms. It forms a vital part of the water cycle, dissolving in oceans and lakes, providing support for aquatic ecosystems.
The Role of Oxygen in Respiration
Respiration, the process by which organisms convert glucose into energy, relies heavily on oxygen. Human beings, for instance, inhale oxygen, which then travels to the lungs and is absorbed into the bloodstream. The oxygenated blood distributes oxygen to tissues and cells, enabling the release of energy from glucose through the process of cellular respiration.
Combustion: A Chemical Reaction Fueled by Oxygen
Combustion, the rapid burning of fuels, is another process that requires oxygen. When a fuel such as wood or gasoline comes into contact with an ignition source and sufficient oxygen, a chain reaction ensues, releasing heat and light. The exothermic nature of combustion drives various applications, from powering engines to generating electricity.
Environmental Impact: Maintaining Balance and Combating Climate Change
Oxygen plays a crucial role in maintaining the Earth’s environmental balance. It is an intrinsic part of the hydrological cycle, supporting marine ecosystems and serving as a determinant of water quality. Moreover, oxygen acts as a sink for carbon dioxide, which helps regulate climate change by absorbing excess amounts of this greenhouse gas.
Oxygen is a fundamental element that sustains life, fuels combustion, and influences environmental dynamics. Its presence in the atmosphere and water bodies is vital for the survival of aerobic organisms. Respiration relies on oxygen to generate energy, while combustion utilizes it to produce heat and light. Oxygen also participates in the regulation of the Earth’s climate, maintaining a delicate balance within the planet’s ecosystems.
Related Concepts: Carbon Dioxide, the Climate’s Invisible Influencer
The concept of carbon dioxide goes beyond mere chemistry; it’s a story of our planet’s history and future. Carbon dioxide, a molecule composed of one carbon atom and two oxygen atoms, is more than just a colorless gas. It plays a pivotal role in photosynthesis, the process that sustains life on Earth, and carries a complex relationship with our climate.
Carbon Dioxide: A Tale of Respiration and Climate
While carbon dioxide is an essential ingredient for photosynthesis, it’s also a product of respiration, the process by which organisms convert food into energy. Every breath we exhale releases this gas into the atmosphere. It’s like a constant, rhythmic dance between plants and animals, swapping life-giving oxygen for carbon dioxide.
However, when human activities, such as burning fossil fuels and deforestation, release excessive amounts of carbon dioxide into the atmosphere, the balance is disrupted. Carbon dioxide acts as a greenhouse gas, meaning it traps heat from the sun, causing the Earth’s temperature to rise. This increase in temperature triggers a cascade of climate effects, including more extreme weather events, rising sea levels, and changes in ecosystems.
The Impact on Our Changing Planet
The impact of carbon dioxide on climate change is undeniable. Scientists have observed a steady increase in carbon dioxide levels in the atmosphere over the past century. This rise is primarily attributed to human activities, such as burning fossil fuels for energy and transportation. The burning of fossil fuels releases large amounts of carbon dioxide into the atmosphere, contributing significantly to climate change.
The consequences of climate change are already being felt across the globe. Rising sea levels are threatening coastal communities, more frequent and intense storms are causing widespread damage, and changes in precipitation patterns are disrupting agricultural practices. The Earth’s climate is changing rapidly, and carbon dioxide is a key driver of these changes.
Understanding Carbon Dioxide: A Path to Climate Solutions
Comprehending the role of carbon dioxide is essential for finding solutions to climate change. By reducing our reliance on fossil fuels, adopting renewable energy sources, and implementing sustainable practices such as tree planting, we can help mitigate the release of carbon dioxide into the atmosphere. By reducing emissions and transitioning to a cleaner, more sustainable energy future, we can protect our planet and ensure a habitable and thriving environment for generations to come.
The Role of Water in Photosynthesis: A Vital Liquid for Life
Water is an indispensable component of photosynthesis, the process that sustains life on Earth. Its presence in this intricate chemical reaction is not merely incidental but plays a crucial role in several ways.
Hydration: The Solvent of Life
Water serves as the solvent for photosynthesis, providing a medium for the chemical reactions to occur. It dissolves various ions, molecules, and proteins involved in the process, enabling them to interact and facilitate the conversion of sunlight into energy.
Source of Hydrogen and Oxygen Atoms
Water’s chemical composition (H2O) makes it a significant source of both hydrogen and oxygen atoms. Hydrogen atoms are incorporated into glucose, the primary energy product of photosynthesis, while oxygen atoms are released as a byproduct. The oxygen released by plants during photosynthesis replenishes the Earth’s atmosphere, providing the essential gas for respiration.
Electron Transport: Unlocking Energy
Water also participates in the electron transport chain, a series of reactions that generate the energy required for photosynthesis. During this process, water molecules donate electrons to chlorophyll molecules, which then facilitate the conversion of light energy into chemical energy.
Regulation and Stability
The presence of water helps regulate the temperature within plant cells during photosynthesis. It acts as a thermal buffer, absorbing excess heat and preventing overheating. Additionally, water provides structural stability to the chloroplasts, the organelles where photosynthesis occurs.
In conclusion, water is not simply a passive component but an active participant in photosynthesis. Its multifaceted role in hydration, providing atoms, facilitating electron transport, and maintaining stability underscores its indispensable nature for sustaining life on our planet.