Phosphorus: A Detailed Overview Of Its Atomic Structure And Properties

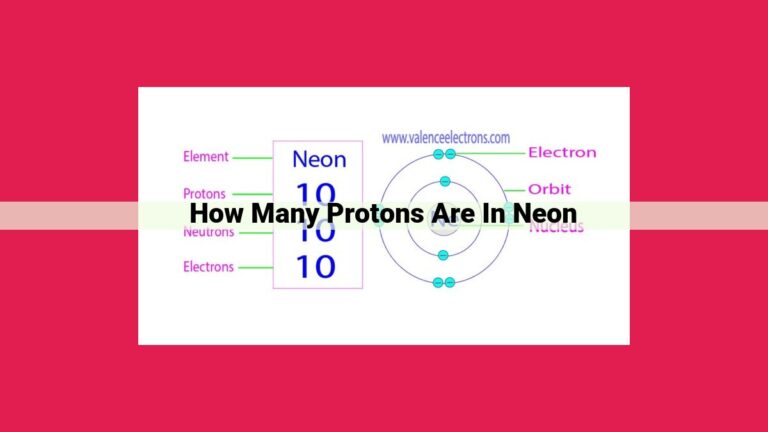
Phosphorus has 15 protons, as determined by its atomic number. The atomic number of an element is the number of protons in its nucleus, which gives the element its identity and distinguishes it from other elements. The nucleus of phosphorus also contains 16 neutrons, giving the element a mass number of 31. The proton number and the atomic number of phosphorus are the same because phosphorus has no electron configuration or isotopes that would alter this relationship.
Phosphorus: Unraveling the Secrets of Its Core
In the realm of science, we constantly seek knowledge and understanding of the intricate building blocks of our world. Today, we embark on a journey to explore the fascinating element phosphorus and unravel the secrets of its atomic core.
Atomic Number: The Unique Identifier
Every element in the periodic table possesses a defining characteristic known as the atomic number. This number represents the number of protons residing in the nucleus, the central core of an atom. Protons carry a positive electrical charge and play a crucial role in determining the unique properties of each element.
Phosphorus: Atomic Number 15
Phosphorus, the element central to our discussion, proudly bears the atomic number 15. This signifies that each phosphorus atom contains 15 protons nestled snugly within its nucleus. These protons, along with the accompanying 16 electrons, establish the unique identity of phosphorus, distinguishing it from all other elements.
Nucleus and Protons
The nucleus serves as the heart of an atom, harboring protons and neutrons. The number of protons within the nucleus is directly influenced by the atomic number. In the case of phosphorus, the atomic number of 15 translates to 15 protons occupying its nucleus.
Proton Number and Atomic Number: A Synonymous Tale
For phosphorus, the terms “proton number” and “atomic number” become synonymous, intertwined in their significance. Both numbers consistently represent the unwavering count of 15 protons within the phosphorus nucleus.
Related Concepts: Expanding Our Understanding
Our exploration extends beyond atomic number to encompass related concepts that provide a deeper understanding of phosphorus.
- Atomic weight represents the total mass of protons and neutrons within the nucleus.
- Neutron number refers to the number of neutrons, particles with no electrical charge, residing in the nucleus.
- Mass number represents the combined number of protons and neutrons in the nucleus.
Phosphorus: A Glimpse into Its Inner Sanctum
In the case of phosphorus, the number of protons in its nucleus profoundly shapes its characteristics and behavior. With 15 protons, phosphorus:
- Belongs to Group 15 (Nitrogen family) of the periodic table
- Exhibits a characteristic valence electron configuration of 3s²3p³
- Forms stable covalent bonds with other elements
- Plays a vital role in biological processes, particularly in the formation of nucleic acids and energy metabolism
Our exploration of the atomic number of phosphorus has unveiled the profound influence of this defining characteristic. Phosphorus, with its atomic number of 15, comprises 15 protons, the cornerstone of its atomic structure. This fundamental understanding provides the basis for further scientific inquiries and technological advancements involving this remarkable element.
Phosphorus Nucleus and Protons: The Heart of Matter
In the realm of chemistry, the study of atoms unravels the fundamental building blocks of our universe. Among these atoms, phosphorus holds a special place, with its unique characteristics and vital role in biological processes. At the heart of every phosphorus atom lies its nucleus, a bustling hub of subatomic particles that dictate its identity and behavior.
The phosphorus nucleus is a compact core, containing a cluster of protons and neutrons. Protons are the positively charged particles that define an atom’s identity by determining its atomic number. In the case of phosphorus, its atomic number is 15, indicating that each phosphorus atom possesses 15 protons within its nucleus. These protons are tightly packed together, forming the central pillar of the atom’s structure.
The number of protons in a nucleus is not arbitrary; it is inextricably linked to the atomic number. The atomic number is a unique identifier for each element, distinguishing it from all others. For phosphorus, its atomic number of 15 signifies that every phosphorus atom consistently carries 15 protons within its nucleus. This fundamental property is what distinguishes phosphorus from other elements, such as nitrogen with 7 protons or sulfur with 16 protons.
The arrangement of protons in the phosphorus nucleus is not haphazard; they are organized in a specific manner to maintain the atom’s stability. The protons are distributed among energy levels, with the lowest energy level being the closest to the nucleus. As protons are added to the nucleus, they occupy higher energy levels, creating a hierarchical structure that governs the atom’s behavior and interactions.
Understanding the number of protons in the phosphorus nucleus is crucial for comprehending its chemical properties. The number of protons determines the element’s reactivity, its ability to form bonds with other atoms, and its overall chemical characteristics. It is the foundation upon which the atom’s identity and behavior rest.
Proton Number vs. Atomic Number: A Phosphorus Perspective
In the realm of atoms, phosphorus stands out as an intriguing element. Understanding its atomic makeup unveils crucial information that sheds light on its properties and behavior.
When delving into the atomic number, we encounter a fundamental aspect of phosphorus. Every element in the periodic table possesses a unique atomic number, which represents the number of protons found within its nucleus. In the case of phosphorus, its atomic number is 15. This implies that every phosphorus atom harbors 15 protons in its core.
The proton number of an element is a term often used synonymously with atomic number. It designates the exact number of protons residing within the nucleus. For phosphorus, the proton number is also 15, mirroring its atomic number. This numerical value serves as a definitive characteristic that sets phosphorus apart from other elements on the periodic table.
It’s worth noting that the proton number, atomic number, and atomic weight are closely intertwined concepts. The atomic weight represents the average mass of all the isotopes of an element, taking into account the number of protons, neutrons, and electrons present. In the case of phosphorus, its atomic weight is approximately 30.97.
As for the neutron number, it signifies the quantity of neutrons within an atom’s nucleus. While the atomic number remains constant for a given element, the neutron number can vary, giving rise to isotopes. Isotopes are variations of an element that share the same atomic number but differ in neutron number and, consequently, in atomic weight.
In summary, the proton number and atomic number of phosphorus are identical, both standing at 15. This fundamental characteristic defines its position on the periodic table and governs its chemical behavior. Understanding these concepts is essential for unraveling the mysteries and properties of phosphorus and other elements that shape our world.
Delving into the Atomic Realm: Unraveling the Proton Mystery of Phosphorus
In the vast labyrinth of the atomic world, each element possesses a unique identity defined by its atomic number. This enigmatic number holds the key to understanding the core structure and behavior of an atom. Let’s embark on a journey to explore the atomic number of phosphorus, unveiling the secrets that lie within its nucleus.
Phosphorus, the essential element that plays a pivotal role in life processes, boasts an atomic number of 15. This number signifies that every phosphorus atom contains 15 protons in its nucleus. Protons, the positively charged particles that reside within the atom’s core, are the driving force behind its electrostatic interactions.
The number of protons in an atom is synonymous with its proton number. In the case of phosphorus, the proton number is also 15. This underscores the direct relationship between atomic number and proton count.
Delving into the Nucleus: Atomic Weight, Neutron Number, and Mass Number
Beyond protons, the nucleus of phosphorus harbors other subatomic particles: neutrons. Unlike protons, neutrons carry no electrical charge, acting as neutral components within the nucleus. The neutron number represents the number of neutrons in the nucleus.
The atomic weight of an element, expressed in atomic mass units (amu), encompasses the combined mass of its protons and neutrons. For phosphorus, the atomic weight is approximately 31 amu, reflecting the sum of its 15 protons and 16 neutrons.
The mass number, another important nuclear concept, represents the total number of protons and neutrons within the nucleus. In the case of phosphorus, the mass number is 31, mirroring its atomic weight.
In conclusion, the number of protons in phosphorus is inextricably linked to its atomic number, which stands at 15. This proton count defines the electrostatic nature of the phosphorus atom and shapes its chemical reactivity. Phosphorus emerges as an element with a rich proton content, rendering it an essential building block for life’s processes.
As we delve deeper into the atomic realm, we unravel the intricate relationship between protons, neutrons, and the atomic structure that governs the behavior of elements like phosphorus and countless others.