Phosphorus: Delving Into The Atomic Number And Its Significance
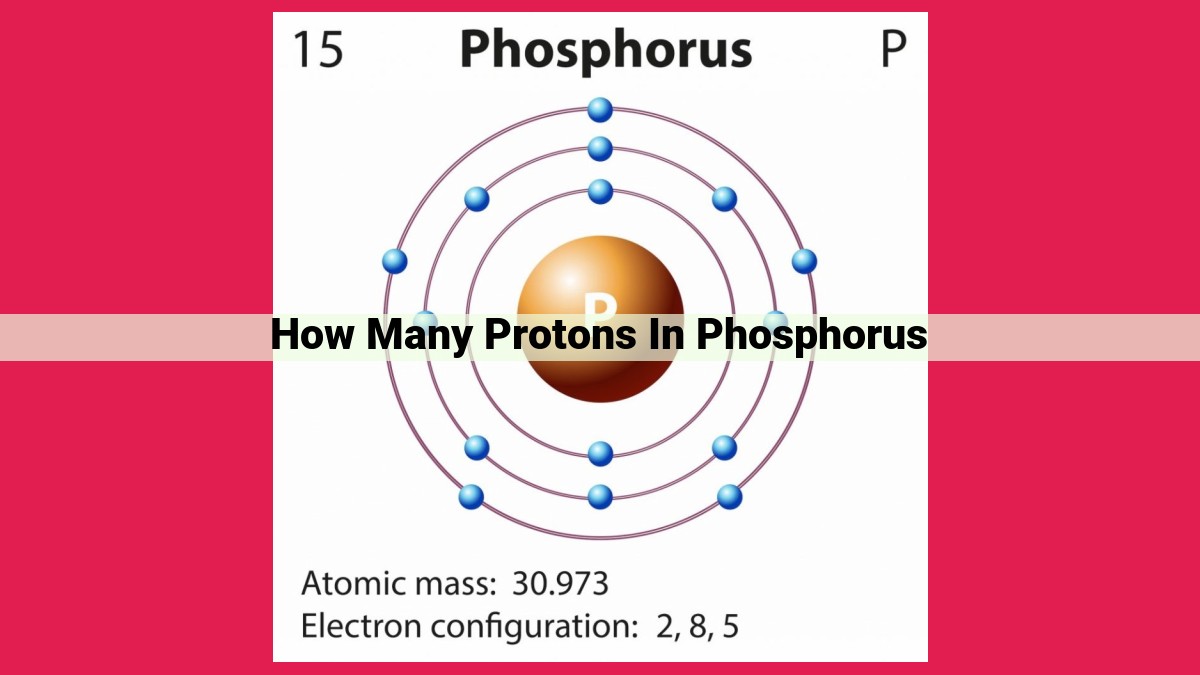
Phosphorus, an essential element, has 15 protons in its nucleus. This number, known as the atomic number, identifies each element uniquely. Protons, positively charged particles, reside in the nucleus along with neutrons. The number of protons determines the element’s chemical identity and distinguishes it from other elements. Understanding the number of protons and other subatomic particles, such as electrons and neutrons, is crucial in unraveling the fundamental building blocks of matter.
Unveiling the Secrets of Phosphorus: A Journey to Its Atomic Core
Phosphorus, an element crucial to life on Earth, plays a pivotal role in countless biological processes. But beyond its significance in the tapestry of life, lies a fundamental question: how many protons reside within its atomic structure? Join us on an enthralling journey to unravel this atomic mystery.
Atomic Number: The Keystone of Element Identification
Every element in the vast tapestry of the periodic table possesses a unique identity defined by its atomic number. This number, a fundamental characteristic, represents the number of protons residing within the nucleus of an atom. Without this atomic compass, the elements would merge into an indistinguishable blur.
Protons: The Positive Guardians of the Nucleus
At the heart of every atom lies the nucleus, a densely packed assembly of protons and neutrons. Protons, with their positive charge, form the very foundation of an atom’s identity. The stark reality is that the number of protons in an atom’s nucleus directly corresponds to its atomic number.
Phosphorus: Atomic Number 15
Phosphorus, an element essential for life, occupies the 15th position on the periodic table. This placement reveals that each phosphorus atom proudly harbors 15 protons within its nucleus. These 15 protons define phosphorus’s unique identity among the elements.
Beyond Protons: Neutrons and Electrons
While protons reign supreme in the nucleus, they share their abode with neutrons, particles devoid of charge. Neutrons, along with protons, contribute to an atom’s mass. Orbiting the nucleus like celestial bodies, electrons, with their negative charge, balance the positive charge of the protons to maintain the atom’s neutrality.
Phosphorus, with its 15 protons, serves as a testament to the fundamental building blocks that underpin the vast tapestry of the universe. Atomic number, protons, neutrons, and electrons, these entities form the cornerstone of chemistry and beyond. Understanding these building blocks empowers us to unravel the mysteries that lie at the heart of matter and life itself.
Atomic Number: The Identity Card of Elements
In the realm of chemistry, where elements dance upon the periodic table, each has a unique code embedded within its very essence—the atomic number. This number, a numerical fingerprint, holds the key to unlocking the identity of an element.
Think of elements as individual characters in a grand play. Just as each actor has a distinct name, every element possesses a one-of-a-kind atomic number. This number represents the count of positively charged particles called protons that reside in the core, or nucleus, of an atom.
The atomic number serves as an unyielding identifier for each element. It distinguishes hydrogen, the lightest of all, with an atomic number of 1, from uranium, a heavyweight with an atomic number of 92. Each element occupies a specific spot on the periodic table, arranged in ascending order of their atomic numbers.
This ingenious ordering system not only organizes elements but also reflects their chemical properties and behaviors. Elements with similar atomic numbers often share physical and chemical characteristics, creating vertical columns on the periodic table known as groups. These groups, such as the alkali metals or the halogens, showcase the periodic trends that guide chemical reactions and shape the world around us.
So, when you look at the periodic table, remember that each element carries its atomic number like a badge of honor. It’s a vital piece of information that allows us to understand the nature of elements and their interactions with each other.
Protons: The Positive Core of Atoms
In the bustling metropolis of atoms, there resides a tiny, yet pivotal entity: the proton. These positively charged subatomic particles form the heart of an atom’s nucleus, the control center where the element’s true identity lies.
Each element that graces the periodic table possesses a unique atomic number, a numerical fingerprint that reveals the number of protons housed within its nucleus. This atomic number is crucial, for it acts as a direct reflection of the proton population.
A Tale of Two Elements
Consider two elements, let’s call them A and B. Element A boasts an atomic number of 3, indicative of the 3 protons that reside in its nucleus. Element B on the other hand, flaunts a grander atomic number of 8, signifying the presence of 8 protons within its nuclear domain.
The Protons’ Influence
The number of protons not only determines an element’s atomic number but also exerts a profound influence on its chemical behavior. The positive charge of protons attracts negatively charged electrons, forming the stable atomic structure we know.
The delicate balance between protons and electrons dictates the element’s reactivity and chemical properties. Atoms with a higher proton count tend to be more chemically reactive, while those with a lower proton count adopt a more inert demeanor.
The Phosphorus Paradox
Take the enigmatic element phosphorus, for instance. This vital nutrient, crucial for life itself, possesses an atomic number of 15. This revelation unveils that every single phosphorus atom harbors 15 protons within its nucleus.
Unraveling the number of protons in an element is not merely an academic pursuit. It’s a testament to the fundamental building blocks that shape the universe around us. From the mundane to the extraordinary, protons play an indispensable role in the symphony of life and chemistry.
Phosphorus: Unraveling the Secrets of Its Atomic Core
In the vast tapestry of chemistry, understanding the fundamentals of elements is paramount. Among these elements, phosphorus stands out as a crucial player, its presence indispensable in myriad biological processes. But how many protons reside within the nucleus of a phosphorus atom, granting it its unique identity? Join us on an exploratory journey to unravel this enthralling enigma.
Atomic Number: A Key to Elemental Identity
Every element that graces the periodic table possesses a unique atomic number, a numerical fingerprint that distinguishes it from all others. This atomic number represents the number of positively charged particles known as protons that reside in the heart of an atom, within its nucleus. Protons, along with their negatively charged counterparts, electrons, define an element’s chemical properties.
Phosphorus: Atomic Number 15
Our focus today, phosphorus, bears the atomic number 15. This fundamental characteristic reveals that each phosphorus atom harbors 15 protons within its nucleus, a defining feature that sets it apart from other elements. This number remains constant across all phosphorus atoms, regardless of their isotopic variations.
Isotopes are different forms of the same element, sharing the same number of protons but varying in the number of neutrons, particles with no electrical charge. While the number of neutrons can fluctuate, the number of protons remains the unwavering anchor of an element’s identity.
Protons: The Pillars of Phosphorus’ Existence
Protons play a pivotal role in shaping an atom’s characteristics. Their positive charge attracts electrons, negatively charged particles that orbit the nucleus, forming a stable electrical balance. The number of protons directly determines the number of electrons, as atoms strive to maintain electrical neutrality.
Understanding the number of protons in an element is fundamental to comprehending its chemical behavior. It governs the element’s position on the periodic table, influencing its reactivity, bonding properties, and place in the grand scheme of chemistry.
Neutrons and Electrons: The Nucleus and Orbiters:
- Briefly mention neutrons as neutral particles in the nucleus.
- Discuss electrons as negatively charged particles that orbit the nucleus.
- Explain that the number of neutrons and electrons can vary among isotopes.
Neutrons and Electrons: The Nucleus and Orbiters
Imagine an atom as a miniature solar system, with a dense, positively charged nucleus at the center, representing the Sun. In this atomic realm, two types of subatomic particles dance around the nucleus like planets: neutrons and electrons.
Neutrons: The Silent Partners
Neutrons, as their name suggests, are electrically neutral particles. They reside within the nucleus alongside protons, contributing to the atom’s overall mass. Unlike protons, neutrons don’t carry an electrical charge, which makes them essential for balancing the positive charges of protons and maintaining the stability of the nucleus.
Electrons: The Orbiting Electrons
Electrons, on the other hand, are negatively charged particles that exist outside the nucleus, occupying various energy levels known as electron shells. These shells resemble concentric circles, with each level holding a specific number of electrons. The outermost electron shell determines the atom’s chemical properties and its ability to interact with other atoms.
Isotopes: Variations on a Theme
Atoms of the same element can have different numbers of neutrons, resulting in variations known as isotopes. Isotopes have the same number of protons and electrons, but differ in their neutron count. For example, phosphorus-31 is an isotope with 15 protons, 16 neutrons, and 15 electrons. Isotopes of the same element share similar chemical properties but can have different radioactive properties or atomic masses.
The Symphony of Subatomic Particles
The harmonious dance of protons, neutrons, and electrons is essential for the very existence of atoms and the world around us. Protons provide the positive charge necessary for chemical bonds, while neutrons stabilize the nucleus and balance the protons’ electrical force. Electrons, with their negative charge, create the outermost shell, determining the atom’s chemical reactivity and shaping the interactions between atoms.
Understanding the roles of these subatomic particles is like deciphering the fundamental language of chemistry, a language that governs the building blocks of our universe.