Phase Changes: Understanding Temperature Constancy With Energy Exchange
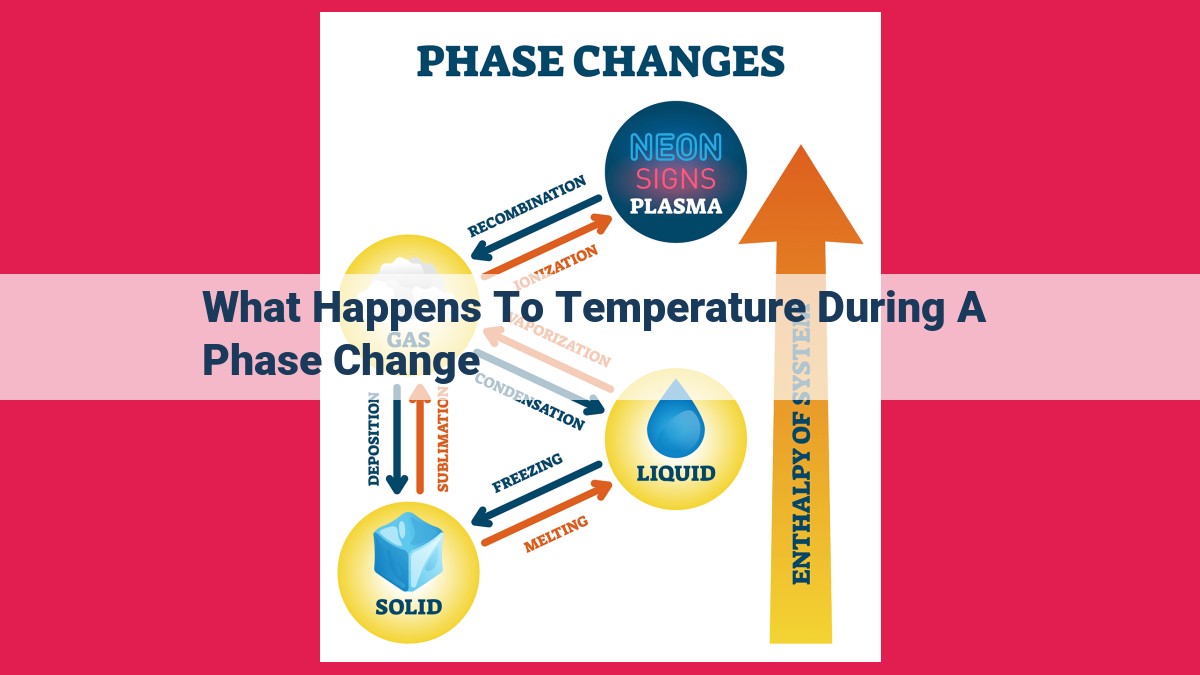
During a phase change, the temperature of a substance remains constant even though energy is being added or removed. This is because the energy is used to break or form intermolecular bonds, not to raise or lower the temperature. For example, when ice melts, the added heat is used to break the bonds between the water molecules, not to heat them up. As a result, the temperature remains at 0°C until all of the ice has melted.
Temperature Changes During Phase Changes: A Journey Through Matter’s Transformations
Have you ever wondered why ice melts at a constant temperature or why water boils at a specific point? These phenomena are not just quirks of nature; they reveal profound insights into the fascinating world of phase changes.
Understanding Phase Changes
Phase changes are the transformations between the three main states of matter: solid, liquid, and gas. Each phase has its unique properties due to the arrangement and bonding of its constituent molecules.
The temperature at which a phase change occurs depends on several factors, including the substance’s chemical composition, pressure, and intermolecular forces. These forces hold molecules together in a particular arrangement, and overcoming them requires a certain amount of energy.
Enthalpy of Fusion and Enthalpy of Vaporization
When a substance undergoes a phase change, it absorbs or releases energy in the form of heat. This energy is known as the enthalpy of fusion or enthalpy of vaporization.
- Enthalpy of Fusion: The energy required to break the intermolecular bonds in a solid and transform it into a liquid.
- Enthalpy of Vaporization: The energy needed to overcome the attractive forces between molecules in a liquid and convert it into a gas.
Melting: Temperature Remains Constant
When a solid melts, it absorbs the enthalpy of fusion. This energy disrupts the tightly packed arrangement of molecules in the solid and allows them to move more freely. However, the temperature remains constant during melting because the absorbed energy is used to break bonds, not to increase the kinetic energy of the molecules.
Vaporization: Temperature Remains Constant
Vaporization is the process by which a liquid transforms into a gas. Similar to melting, vaporization requires energy in the form of the enthalpy of vaporization. Once again, the absorbed energy is used to overcome intermolecular forces, allowing the molecules to escape the liquid and form a gaseous state. Thus, the temperature also remains constant during vaporization.
Constant Temperature During Phase Changes
The key takeaway is that temperature only changes when the kinetic energy of molecules changes. During phase changes, the added or removed energy is used for phase transformation, not temperature changes. The temperature remains constant because the energy is used to disrupt the intermolecular forces holding the molecules in a particular arrangement.
Enthalpy of Fusion and Enthalpy of Vaporization: The Energy Behind Phase Changes
Introduction
Phase changes, the transformations between solid, liquid, and gas, are captivating physical processes that play a crucial role in our daily lives. From the melting of ice to the boiling of water, these changes involve the absorption or release of energy, which is captured by two important concepts: enthalpy of fusion and enthalpy of vaporization.
Enthalpy of Fusion
Enthalpy of fusion, denoted by ΔHfus, refers to the amount of energy required to transform one mole of a solid into a liquid at its melting point. When a solid melts, ΔHfus is essential in overcoming the intermolecular forces that hold the solid particles together. This energy input often manifests as an increase in temperature and a change in molecular motion, leading to the transition from a rigid solid to a fluid liquid.
Enthalpy of Vaporization
Enthalpy of vaporization, denoted by ΔHvap, represents the energy required to convert one mole of a liquid into a gas at its boiling point. Similar to ΔHfus, ΔHvap drives the process of vaporization by breaking the intermolecular forces in the liquid. However, vaporization requires a significantly greater amount of energy compared to melting as the liquid molecules must overcome not only the intermolecular forces but also the atmospheric pressure. This substantial energy input translates into a rapid increase in temperature and a dramatic change in molecular motion, transforming the liquid into a gaseous state.
Melting: An Unchanging Temperature Journey
When we encounter a piece of ice, we marvel at its solid form, held together by a rigid embrace of intermolecular bonds. As we provide warmth to the ice, something intriguing occurs. It begins to melt, transforming from a rigid solid to a flowing liquid. During this transition, a peculiar phenomenon emerges: the temperature of the ice remains constant.
Understanding the Melting Process
Melting, a type of phase change, is a process where a solid absorbs heat energy, causing its molecules to break free from their tightly bound arrangement. This process occurs when the temperature of the solid reaches its melting point, a specific temperature unique to each substance.
The Role of Intermolecular Bonds
The intermolecular bonds within a solid are like tiny glue molecules, holding its structure intact. As heat is added, these bonds begin to weaken, allowing the molecules to move more freely. However, melting is not simply a matter of breaking bonds and letting the molecules go their own way. The energy from the heat is not used to increase the temperature but rather to overcome the force of the intermolecular bonds.
Constant Temperature at the Melting Point
As the solid continues to absorb heat, more and more molecules break free from the intermolecular bonds. However, the temperature remains constant at the melting point. This is because the energy provided is not used to increase the temperature but to facilitate the phase change from solid to liquid. The absorbed heat goes directly into breaking the bonds, not into raising the temperature.
Significance of Constant Temperature
The constant temperature during melting has important implications. It means that all the heat energy provided is used for the phase change, ensuring that the entire solid melts before the temperature starts to rise. This phenomenon helps us accurately determine the melting point of a substance and understand its properties.
By understanding the process of melting, we gain insights into the fundamental nature of matter and the delicate balance between intermolecular forces and temperature. The next time you witness ice melting, remember the captivating dance of molecules, their journey from a rigid embrace to a flowing freedom, all while maintaining a constant temperature.
Vaporization: Temperature Remains Constant
Embark on a Journey of Understanding Phase Changes
In the realm of matter, substances undergo transformations between three distinct phases: solid, liquid, and gas. One of these transformations is vaporization, the transition from a liquid to a gas. As you embark on this journey, you’ll unravel the secrets of why temperature remains constant during vaporization.
Overcoming Intermolecular Forces
Imagine a crowd of molecules, tightly packed together in liquid form. To break free and transform into a gas, these molecules need to overcome the intermolecular forces holding them in place. This is where heat comes into play. As heat is added to the liquid, the molecules absorb it, gaining energy and breaking away from their liquid confines.
The Boiling Point: A Temperature Threshold
As you continue to add heat, you reach a critical point known as the boiling point. At this temperature, the intermolecular forces can no longer hold the molecules back. They surge toward the surface, overcoming the atmospheric pressure and escaping into the gaseous realm.
Temperature Constancy: A Phase Change Phenomenon
Throughout the vaporization process, an intriguing phenomenon occurs: the temperature remains constant. Unlike other heating processes, where temperature rises with added heat, vaporization defies this expectation. The reason lies in the energy distribution.
The heat added during vaporization does not cause an increase in temperature. Instead, it is absorbed by the molecules for phase transformation, overcoming intermolecular forces and transforming the liquid into a gas. This energy is used to break molecular bonds, not to raise the temperature.
In vaporization, temperature constancy is a testament to the remarkable nature of phase changes. As molecules break free from liquid bonds, they absorb energy without raising the overall temperature. Vaporization serves as a reminder that temperature changes are not always synonymous with energy transfer, but rather, specific to the phase transformation process.
Temperature Constant During Phase Changes: A Tale of Energy Transformation
When matter undergoes a phase change, it transforms from one state (solid, liquid, or gas) to another. During these transitions, a remarkable phenomenon occurs: the temperature remains constant. This fascinating observation is a testament to the intricate interplay of energy and molecular interactions.
Phase changes are not merely physical transformations; they involve the exchange of energy. The energy required to break the bonds between molecules during melting (solid to liquid) or vaporization (liquid to gas) is known as enthalpy of fusion and enthalpy of vaporization, respectively.
As heat is added to a solid, its temperature initially increases. However, once the melting point is reached, the added heat is no longer used to raise the temperature. Instead, it is absorbed by the molecules, overcoming the intermolecular forces that hold them in a fixed lattice structure. This energy absorption allows the molecules to transition from a rigid, ordered arrangement to a more fluid state, without a noticeable change in temperature.
Similarly, when a liquid is heated, its temperature gradually increases until the boiling point. At this point, the added heat is again absorbed, but this time it is used to break the intermolecular forces that keep the molecules closely packed. As molecules absorb this energy, they gain enough kinetic energy to escape from the liquid’s surface and transition into a gaseous state. Again, the temperature remains constant at the boiling point, as the added heat is consumed by the phase transformation process.
The constancy of temperature during phase changes underscores the concept of latent heat. This energy is hidden, stored within the molecules, and is not reflected in the temperature of the substance. The added heat during melting or vaporization is used to overcome molecular interactions, not to increase the temperature.
In summary, the temperature remains constant during phase changes because the added energy is utilized for phase transformation, rather than increasing the kinetic energy of the molecules. This phenomenon highlights the importance of energy in driving molecular rearrangements and the unique properties of matter in different phases.
Endothermic and Exothermic Phase Changes
When a substance undergoes a phase change, such as melting, freezing, vaporization, or condensation, its temperature remains constant. This is because the energy added or removed during these processes is used to break or form intermolecular bonds, not to increase or decrease the temperature.
-
Endothermic Phase Changes: In endothermic phase changes, energy must be added to the substance to cause the change. For example, when ice melts, heat must be added to break the hydrogen bonds between the water molecules. This heat is absorbed by the ice, so the temperature of the ice does not increase as it melts. Other examples of endothermic phase changes include vaporization (liquid to gas) and sublimation (solid to gas).
-
Exothermic Phase Changes: In exothermic phase changes, energy is released by the substance as it undergoes the change. For example, when water freezes, the hydrogen bonds between the water molecules form, releasing heat. This heat is released into the surroundings, so the temperature of the water decreases as it freezes. Other examples of exothermic phase changes include condensation (gas to liquid) and deposition (gas to solid).
In summary, endothermic phase changes require the addition of energy, while exothermic phase changes release energy. These phase changes play important roles in various natural and industrial processes, affecting the behavior and properties of substances in our environment.