Ph Impact On Solubility: Understanding Intermolecular Forces And Equilibria For Enhanced Predictions
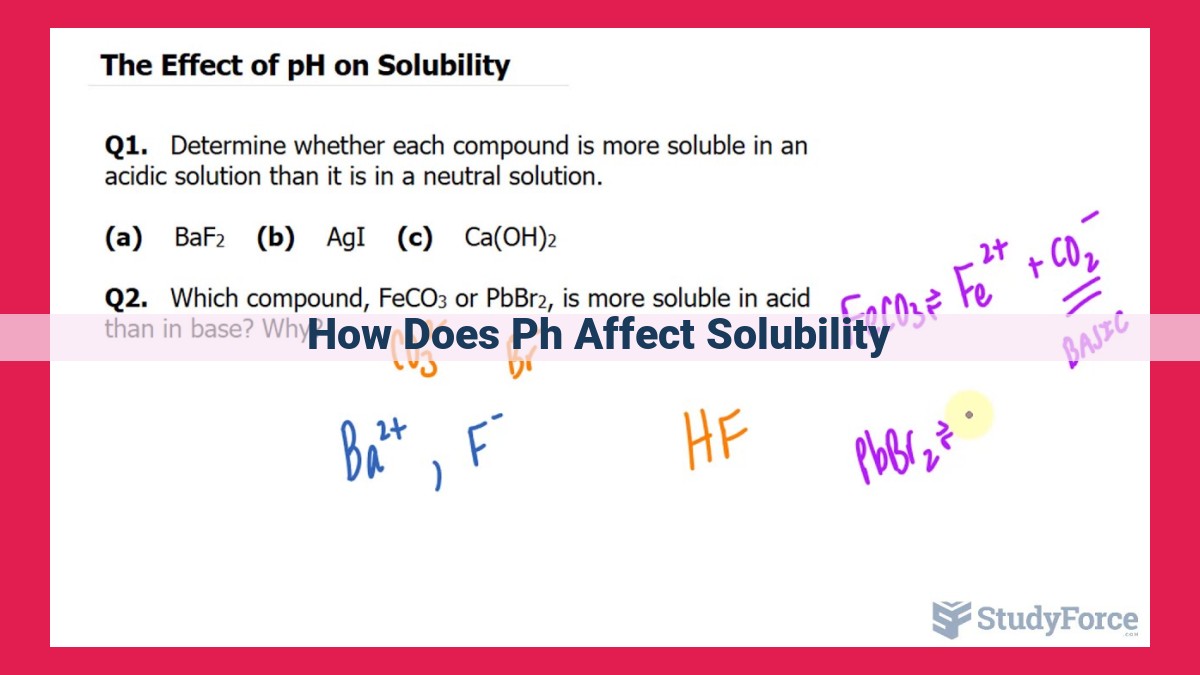
pH significantly impacts solubility due to its influence on intermolecular forces. Acidic and basic solutions alter the solubility of solutes based on their polarity and interaction with the solvent. Ionization and chemical equilibrium further play a role, with strong electrolytes affecting solubility differently compared to weak electrolytes. Le Chatelier’s principle helps predict solubility changes under varying conditions. The common ion effect and solubility product constant (Ksp) determine precipitation and dissolution equilibria, influenced by factors like supersaturation, surface area, and temperature.
The Alchemy of pH and Solubility: Unraveling the Secrets of Chemical Reactions
In the intriguing world of chemistry, the concepts of pH and solubility play pivotal roles in unraveling the secrets of countless chemical reactions. Let’s embark on a captivating journey to comprehend their significance.
pH: The Acidity-Basicity Enigma
pH, a ubiquitous measure in chemistry, serves as a window into the acidity and basicity of solutions. When the pH value of a solution is below 7, it’s acidic, indicating an abundance of hydrogen ions (H+). Conversely, solutions with pH values above 7 are basic, characterized by a higher concentration of hydroxide ions (OH-).
The pH of a solution has a profound impact on chemical reactions. For instance, in an acidic environment, reactions favoring the formation of products with a higher positive charge are enhanced. Conversely, in a basic environment, reactions producing products with a higher negative charge are more likely to occur.
Solubility: The Dance of Molecules and Solvents
The solubility of a solute in a solvent hinges on the strength of intermolecular forces. Molecules with strong intermolecular forces, such as dipole-dipole interactions or hydrogen bonds, prefer to remain in their liquid or solid state and may have lower solubility in a given solvent. In contrast, molecules with weaker intermolecular forces tend to dissolve more readily.
This principle is particularly relevant in separating mixtures. For instance, organic compounds with nonpolar molecules are typically soluble in nonpolar solvents like hexane. On the other hand, polar or ionic compounds exhibit higher solubility in polar solvents like water due to stronger intermolecular forces.
Unveiling the Symphony of Ionization and Chemical Equilibrium
Ionization, the process of forming ions from atoms or molecules, plays a crucial role in chemical reactions. Electrolytes, substances that conduct electricity when dissolved in water, undergo ionization to various degrees. Strong electrolytes, such as acids and bases, ionize completely, while weak electrolytes ionize only partially.
Ionization and chemical equilibrium are intricately linked. Chemical reactions strive to reach a state of equilibrium, where the forward and reverse reactions occur at equal rates. This delicate balance can be shifted by manipulating the concentration, temperature, or other factors according to Le Chatelier’s principle.
Acid-Base Reactions: The Dance of Protons
Acid-base reactions, the heart of many chemical interactions, involve the transfer of protons (H+). Acids donate protons, while bases accept them. The process of neutralization occurs when acids and bases react, forming salts and water.
Solubility Product and Precipitation: The Art of Dissolution and Crystallization
The solubility product constant (Ksp) is a critical concept in understanding precipitation and dissolution. Ksp represents the equilibrium constant for the dissolution of a solid in a solvent, providing insights into the solubility of ionic compounds.
When the concentration of ions in a solution exceeds the Ksp value, precipitation occurs, forming a solid. Conversely, dissolution takes place when the concentration of ions is below the Ksp value.
Epilogue: The Significance of pH and Solubility in the Chemical World
The concepts of pH and solubility are fundamental pillars in chemistry, guiding our understanding of countless chemical reactions. From the acidity of solutions to the solubility of substances, these concepts are essential in diverse fields such as chemistry, biology, and environmental science. By unraveling their mysteries, we gain a deeper appreciation for the intricate harmony that governs the chemical world.
Hydrogen Ion Concentration and pH:
- Define pH and explain its connection to hydrogen ion concentration.
- Describe how to identify acidic, basic, and neutral solutions based on their pH values.
Hydrogen Ion Concentration and pH: The Key to Understanding Acid-Base Chemistry
In the realm of chemical reactions, the concept of pH plays a crucial role in determining the acidity or basicity of a solution. Hydrogen ion concentration is the underlying factor that dictates this all-important value.
pH: The Numerical Representation of Acidity
pH is a logarithmic scale that measures the concentration of hydrogen ions (H+) in a solution. The lower the pH value, the higher the concentration of hydrogen ions, indicating a more acidic solution. Conversely, higher pH values denote lower hydrogen ion concentrations, indicating a more basic solution. Neutral solutions, with a pH of 7, have equal concentrations of hydrogen and hydroxide ions, indicating a balanced state.
Identifying Acidic, Basic, and Neutral Solutions
Determining the pH of a solution is essential for understanding its chemical behavior. Acidic solutions have pH values below 7, basic solutions have pH values above 7, while neutral solutions have a pH of 7. Knowing the pH value of a solution allows scientists to predict its reactivity and make informed decisions about its use.
Hydrogen Ion Concentration: The Driving Force Behind pH
The relationship between pH and hydrogen ion concentration is direct and inversely proportional. As hydrogen ion concentration increases, pH decreases, and vice versa. Understanding this relationship is vital for manipulating solutions and controlling chemical reactions.
Hydrogen ion concentration and pH are fundamental concepts in chemistry, providing a foundation for comprehending the behavior of acids, bases, and chemical reactions. By understanding the numerical representation of acidity and the underlying role of hydrogen ions, scientists can delve deeper into the complexities of chemical interactions and unlock the potential of chemistry for countless applications.
Intermolecular Forces: The Invisible Glue that Determines Solubility
In the intricate world of chemistry, the ability of substances to dissolve and interact is governed by intermolecular forces—the invisible connections that exist between molecules. These forces play a pivotal role in determining the solubility of a solute in a particular solvent.
Types of Intermolecular Forces
Intermolecular forces can take various forms, each contributing to the solubility behavior of a substance. The dipole-dipole force arises when polar molecules possess a permanent separation of charge, creating a positive end and a negative end. These molecules align and interact with each other, forming a weak bond.
The hydrogen bond is a particularly strong dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen or nitrogen. This results in a strong attraction between the hydrogen atom in one molecule and the electronegative atom in another.
Influence on Solubility
The type and strength of intermolecular forces influence the solubility of a solute in a solvent. “Like dissolves like” is a fundamental principle in chemistry. Substances with similar intermolecular forces tend to be soluble in each other.
For instance, polar solutes, such as salt or sugar, form strong dipole-dipole interactions with polar solvents like water. The water molecules orient themselves around the polar solute, shielding it from the surrounding water molecules and enabling it to dissolve.
Conversely, nonpolar solutes, such as oil or grease, lack permanent charge or polarity. They experience weak van der Waals forces that are insufficient to overcome the polar interactions in water. As a result, nonpolar solutes are generally insoluble in polar solvents.
Ionization and Chemical Equilibrium: A Tale of Balance
Ionization: The Key to Electrolytes
Imagine a party where everyone is dancing freely. This is ionization, where neutral atoms or molecules split into ions, carrying a positive or negative charge. These charged ions are like the life of the party, creating an “electric” atmosphere. They’re what make electrolytes, like salt water, conduct electricity.
Strong and Weak Electrolytes: A Tale of Two Classes
Not all electrolytes are created equal. Strong electrolytes are like the extroverted party guests who dissociate completely into ions, leaving no neutrals behind. They’re highly sociable and make their presence known. On the other hand, weak electrolytes are more introverted. They only partially dissociate, preferring to hang out in a neutral state.
Acids, Bases, and pH: The Balancing Act
Acids and bases are like opposing forces in the chemical world. Acids release hydrogen ions (H+), while bases release hydroxide ions (OH-). The balance between these ions determines a solution’s pH, which is a measure of its acidity or basicity. A low pH indicates a high concentration of H+, making the solution acidic. A high pH means more OH-, resulting in a basic solution.
Equilibrium: The Dance of Reversibility
Chemical reactions aren’t always one-way streets. Some reactions can run in both directions, like a never-ending dance. This is called reversible reactions and chemical equilibrium is the delicate balance point where the forward and reverse reactions occur at equal rates.
Le Chatelier’s Principle: The Balancing Waltz
Like a skilled choreographer, Le Chatelier’s principle helps us predict how chemical equilibrium will shift when we change the conditions of the system. If we increase the concentration of a reactant, the equilibrium will shift to the product side to balance it out. Similarly, if we add a product, the equilibrium will shift to the reactant side.
Acid-Base Reactions and Le Chatelier’s Principle
In the realm of chemistry, acids and bases engage in fascinating dance, exchanging protons like partners in a waltz. These reactions, known as acid-base reactions, play a pivotal role in shaping the world around us. Imagine the fizz of a carbonated beverage, the sting of vinegar on your tongue, or the acidic rain that falls upon the earth—all orchestrated by these remarkable chemical interactions.
One of the most fundamental aspects of acid-base reactions is the neutralization process. When an acid and a base come together, they react to form a salt and water. This reaction is like a chemical truce, where the acidic and basic properties cancel each other out, creating a solution that is neither acidic nor basic.
But what happens when we disturb this delicate equilibrium? Enter Le Chatelier’s principle, a guiding force in chemistry that predicts how a system will respond to changes in its environment. According to this principle, if you stress a system at equilibrium, it will shift in a direction that relieves that stress.
Let’s say we add more acid to a neutral solution. The system will shift to counteract this change by increasing the concentration of base. Similarly, if we add more base, the system will shift to produce more acid. This phenomenon is like a chemical game of tug-of-war, where the system strives to maintain a state of balance.
Example: Consider the reaction between acetic acid (CH3COOH) and sodium hydroxide (NaOH):
CH3COOH + NaOH → CH3COONa + H2O
If we add more acetic acid to this solution, the equilibrium will shift to the right, producing more sodium acetate (CH3COONa) and water. This is because the system is trying to reduce the excess acetic acid in the solution.
Le Chatelier’s principle is an invaluable tool for predicting the behavior of chemical systems. By understanding how equilibrium shifts occur, we can manipulate reactions to achieve desired outcomes, whether it’s optimizing industrial processes or simply understanding the chemistry of everyday life.
Common Ion Effect and Solubility Product Constant:
- Explain the common ion effect and how it suppresses ionization and reduces solubility.
- Define the solubility product constant (Ksp) and discuss its applications in predicting precipitation and dissolution.
Common Ion Effect and Solubility Product Constant
Understanding the behavior of ions in solution is crucial in chemistry, and the common ion effect and solubility product constant (Ksp) are two fundamental concepts that shed light on the interplay between ion concentration and solubility.
Common Ion Effect
Imagine a crowded party with too many guests for the space available. When you add more guests who are very similar to the ones already present, it becomes increasingly difficult for these guests to find space to dance. In chemistry, this is analogous to the common ion effect. When you add a common ion, such as sodium (Na+) to a solution that already contains it, the ionization of the solute is suppressed. This is because ions of the same charge repel each other, reducing their ability to dissolve and form ions.
Solubility Product Constant (Ksp)
The solubility product constant is a constant value that determines the maximum solubility of an ionic compound in a particular solvent at a specific temperature. It is expressed as:
Ksp = [Cation+]^x [Anion+]^y
where:
- [Cation+] is the molar concentration of the cation
- [Anion+] is the molar concentration of the anion
- x and y are the stoichiometric coefficients of the cation and anion in the ionic compound
The Ksp is a quantitative measure of the solubility of an ionic compound. If the ion product, [Cation+]^x [Anion+]^y, exceeds the Ksp value, the solution is supersaturated, and the compound will precipitate out. Conversely, if the ion product is less than the Ksp, the solution is unsaturated, and the compound will dissolve.
Applications of Ksp
The Ksp has numerous applications in chemistry, including:
- Predicting precipitation and dissolution
- Determining the solubility of ionic compounds
- Understanding the effect of pH on ion concentrations
- Designing experiments to control precipitation and dissolution processes
By understanding the common ion effect and the solubility product constant, scientists can gain valuable insights into the behavior of ions in solution and control chemical reactions involving precipitation and dissolution.
Precipitation and Dissolution:
- Describe the process of precipitation, including the role of supersaturation and factors that influence it.
- Explain the concept of solubility equilibrium and the establishment of dissolution.
- Discuss factors that affect the rate of dissolution, such as surface area and temperature.
Precipitation and Dissolution: A Journey of Transformation
In the realm of chemistry, precipitation and dissolution orchestrate a captivating dance of change. Precipitation, a process of metamorphosis, transforms a solute into an insoluble solid, while dissolution, its antithesis, liberates the solid back into solution.
The Alchemy of Precipitation
When a solution becomes supersaturated with a solute, the stage is set for precipitation. Like a crowded dance floor, the solvent molecules can no longer accommodate the excess solute particles, forcing them to seek refuge in a more stable state. These particles collide and coalesce, forming microscopic crystals that grow and aggregate until visible precipitates emerge.
Factors such as temperature, concentration, and the presence of impurities influence the process of precipitation. By tweaking these parameters, chemists can control the size, shape, and purity of the resulting precipitates, a skill crucial in various industries, from pharmaceuticals to metallurgy.
The Dissolving Act: A Return to Solution
Dissolution, the inverse of precipitation, is a process of transformation in which a solid solute embarks on a journey back into solution. This occurs when the solvent molecules regain their ability to accommodate the solute particles, dissolving their bonds and releasing them into the liquid phase.
Solubility equilibrium, a delicate balance between precipitation and dissolution, determines the fate of the solute. This equilibrium is influenced by factors such as temperature, surface area, and particle size. Increasing the temperature or surface area promotes dissolution, while decreasing particle size enhances the rate of dissolution.
Applications Galore
Precipitation and dissolution are not only fascinating phenomena but also find practical applications in various fields. In water purification, precipitation helps remove impurities from water, while in metallurgy, it facilitates the extraction and purification of metals. Dissolution plays a vital role in pharmaceuticals, as it enables the delivery of drugs in a soluble form, and in food chemistry, it governs the extraction and preservation of nutrients.
By understanding the intricacies of precipitation and dissolution, we gain a deeper appreciation for the dynamic nature of chemical systems and their relevance to our daily lives.