Discover The Periodic Table’s Periods: Uncover The Energy Levels And Electron Relationships
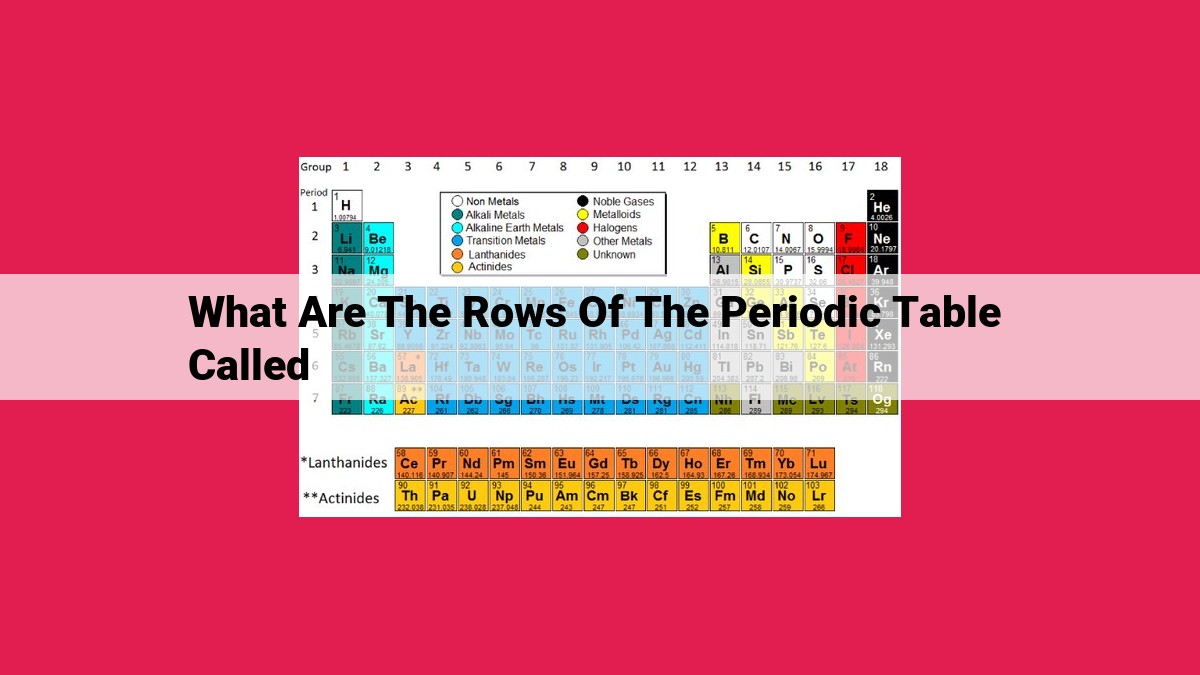
The rows of the periodic table are called periods. They represent the energy levels of electrons in atoms. Each period corresponds to a specific number of energy levels, and elements in the same period have the same number of energy levels but may differ in electron count. Periods are significant in organizing and classifying elements, as they establish relationships and patterns among them.
- Brief introduction to the periodic table and its significance in chemistry
- Define the term “period”
Understanding the Periodic Table: A Journey Through Its Horizontal Rows
In the realm of chemistry, the periodic table stands as a testament to the order and organization of the elements that form the building blocks of our universe. Its vertical columns, known as groups, reveal patterns based on shared chemical properties. But it’s the horizontal rows of the table, the periods, that provide a unique perspective on the energy levels of electrons.
A period is a row that runs horizontally across the periodic table. Each period represents a distinct energy level for electrons within an atom. As we move from left to right across a period, the atomic number increases, indicating an increase in the number of protons within the nucleus. This, in turn, attracts more electrons to occupy the available energy levels.
The number of energy levels within a period determines how many electrons can be accommodated. The first period has only one energy level, while subsequent periods have two, three, and so on. Elements in the same period share the same number of energy levels, but differ in the number of electrons they possess.
Take hydrogen and helium, the first two elements in the periodic table. Both belong to the first period, meaning they have a single energy level. Hydrogen has just one electron, while helium has two. This difference in electron count gives rise to their distinct chemical properties.
The significance of periods extends beyond the organization of elements. They establish relationships and patterns among the elements, helping us to predict their properties and behaviors. Understanding periods is essential for deciphering the periodic table and unlocking the secrets it holds.
Periods: The Horizontal Rows of the Periodic Table
- Explain that periods run horizontally across the table
- Describe how periods represent the energy levels of electrons in atoms
Periods: The Horizontal Rows in the Periodic Table
Embark on a captivating journey through the periodic table, an invaluable roadmap that unveils the secrets of chemistry. Understanding its structure empowers us to decipher the mysteries of the atomic world.
Navigating the Horizontal Rows
Imagine the periodic table as a grand tapestry, woven with elements arranged in horizontal rows known as periods. These periods are like celestial pathways that lead us through the energy levels of electrons in atoms. As we traverse from one period to the next, we ascend an energy ladder, where each period represents a distinct rung.
Energy Level Symphony
Within each period, the elements share a common trait: the number of energy levels occupied by their electrons. These energy levels, like cosmic orbits, encircle the atom’s nucleus. In period 1, for instance, both hydrogen and helium possess just one energy level, albeit with varying numbers of dancing electrons.
As we progress through higher periods, the number of energy levels increases, creating a hierarchy of electron orbits. This arrangement influences the chemical properties of the elements, paving the path for their diverse roles in the symphony of nature.
The Number of Energy Levels and Electron Distribution
Each period within the periodic table corresponds to a specific number of energy levels that surround the atom’s nucleus. These energy levels are often visualized as concentric circles, with the nucleus at the center. Each energy level has a certain capacity for holding electrons, which are negatively charged particles that orbit the nucleus.
Elements within the same period share the same number of energy levels, but they differ in the number of electrons they possess. For example, in the first period, both hydrogen and helium have one energy level, but hydrogen has one electron, while helium has two.
As we move across a period from left to right, the number of electrons steadily increases. This is because the atomic number (the number of protons in the nucleus) also increases from left to right. Protons have a positive charge, and electrons have a negative charge. Therefore, the number of electrons must increase to balance the increasing positive charge of the nucleus.
In summary, the number of energy levels in an atom is determined by its period, while the number of electrons in an atom is determined by its position within the period.
Understanding Periods: The Horizontal Rows of the Periodic Table
The periodic table, a cornerstone of chemistry, is a masterful arrangement of elements based on their atomic properties and characteristics. It’s a treasure trove of knowledge, organizing the chemical world into a logical and comprehensible structure. Periods, the horizontal rows within the table, play a crucial role in this organization.
Periods: A Window into Electron Energy Levels
Periods are the horizontal rows that run across the periodic table. They represent the energy levels of electrons within atoms. As you move from left to right within a period, the atomic number of each element increases, which corresponds to an _increase in the number of protons and electrons_.
Energy Levels and Electron Distribution
Each period corresponds to a specific number of energy levels. The first period has one energy level, the second period has two, and so on. Elements within the same period have the same number of energy levels but may differ in their electron count. This difference in electron count gives rise to the unique properties of each element.
Period 1: Hydrogen and Helium
Period 1, the first row of the periodic table, consists of just two elements: hydrogen and helium. Both hydrogen and helium possess a single energy level, but they differ in their electron count. Hydrogen has one electron, while helium has two. This difference in electron number accounts for their distinct chemical behaviors and properties.
Significance of Periods in the Periodic Table
Periods are essential for organizing and classifying elements. They establish relationships and patterns among the elements. By studying the periods, scientists can predict the properties of an element based on its position within the table. The periodic table becomes a powerful tool for understanding the behavior and interactions of elements.
The Significance of Periods in the Periodic Table
In the realm of chemistry, the periodic table is a treasure trove of knowledge, providing a systematic arrangement of the elements that unveils their remarkable patterns and relationships. Among the table’s key features are the periods, which run horizontally across the grid and play a crucial role in understanding the properties and behaviors of elements.
Organizing and Classifying Elements
The periodic table’s periods serve as an organizational framework for the elements. Elements within the same period share a common trait: they all have the same number of energy levels. This fundamental characteristic dictates the number of electrons an element can hold and influences its chemical reactivity. By grouping elements based on their energy level structure, the periodic table enables chemists to identify similarities and differences among them, making it easier to predict their properties and reactions.
Establishing Relationships and Patterns
The arrangement of elements within the periods reveals fascinating patterns and relationships. As you move across a period from left to right, you encounter elements with an increasing atomic number. This corresponds to a gradual increase in the number of protons and electrons, which in turn affects the element’s chemical characteristics. For instance, elements in the second period (e.g., lithium, beryllium, boron) have two energy levels, but they vary in their number of valence electrons, leading to distinct chemical properties.
Moreover, the periodic table’s periods provide insights into the outermost electron configuration of elements. Elements in the same period have electrons in the same energy level, but their distribution may differ. This variation in electron configuration influences the element’s chemical bonding behavior and its position on the electronegativity scale. By understanding the patterns within periods, chemists can gain valuable information about the reactivity, stability, and other properties of different elements.