Comprehensive Guide To The Periodic Table: Unveiling Element Properties And Chemical Reactivity
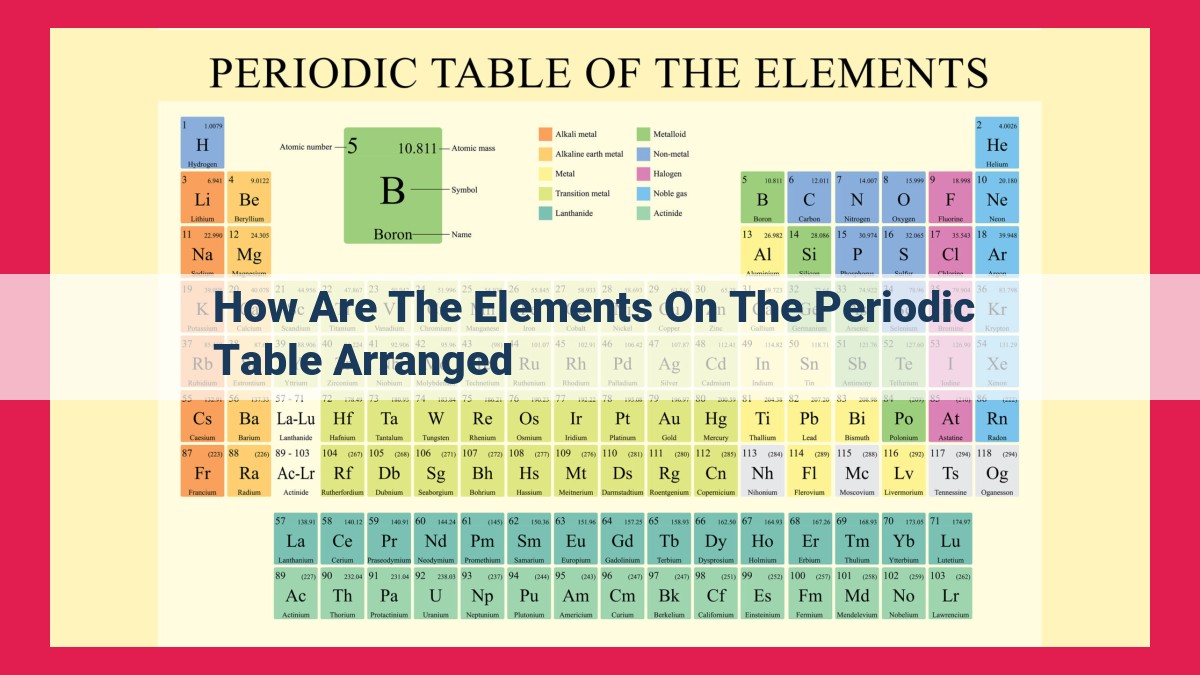
The periodic table arranges elements based on their atomic number, which is the number of protons in the nucleus. This number determines the element’s identity and properties. Elements are arranged horizontally in periods, which represent the number of electron energy levels, and vertically in groups, which represent the number of valence electrons (outermost electrons). Valence electrons play a crucial role in chemical bonding and reactivity, and elements in the same group tend to exhibit similar properties. The noble gas configuration, with a full set of valence electrons, represents a stable state that all elements strive for. The periodic table is a valuable tool for organizing and understanding the chemical elements and their properties.
The Atomic Number: The Essence of an Atom’s Identity
At the heart of every atom, a fundamental characteristic governs its very essence: the atomic number. This enigmatic number represents the number of protons, positively charged particles that reside in the nucleus, the atom’s central core. Protons, along with neutrons, electrically neutral particles, form the bulk of an atom’s mass.
Like a cosmic dance, nucleons – the collective term for protons and neutrons – determine the unique identity of an element. Imagine a celestial orchestra, where protons contribute their positive charge, and neutrons provide a steady harmonic balance. The atomic number, symbolized by the letter Z, becomes the conductor of this symphony, defining the element’s position on the periodic table, the grand map of all known elements. This number is what distinguishes hydrogen, helium, carbon, and all their brethren from one another.
Delving into the Atomic Realm: Uncovering the Secrets of Elements
Every element that makes up our world, from the air we breathe to the devices we use daily, possesses a unique identity defined by its atomic number. Imagine an atom’s nucleus as the heart of the atom, containing positively charged protons, the fundamental building blocks that determine an element’s identity. The number of protons in an atom’s nucleus is its atomic number, a crucial factor in shaping its chemical behavior and properties.
Accompanying the protons in the nucleus are neutrons, particles without an electrical charge. Together with protons, neutrons contribute to the atomic mass of an atom. Understanding atomic number and atomic mass provides a foundation for comprehending the diverse elements that make up the universe. These fundamental properties lay the groundwork for unraveling the intricate patterns and relationships that govern the chemical world.
Define atomic mass as the sum of proton and neutron masses in an atom.
Understanding the Atomic Number: The Foundation of Elemental Identity
Atomic mass is the sum of the masses of all the protons and neutrons in an atom’s nucleus. Protons carry a positive charge, while neutrons are uncharged. The atomic number, on the other hand, is the number of protons in an atom’s nucleus. It’s a unique identifier for each element, determining its chemical properties.
Atomic Mass: The Sum of the Building Blocks
Imagine atoms as tiny building blocks, with protons and neutrons as their core components. The atomic mass is the combined weight of these building blocks. This mass helps distinguish between different isotopes of the same element. Isotopes are atoms of the same element that have the same atomic number but different numbers of neutrons.
Periodic Trends: Uncovering the Properties’ Patterns
The properties of elements show predictable patterns when arranged according to their atomic numbers. This is known as periodic trends. As we move across a period (horizontal row) from left to right, the atomic number increases, leading to changes in atomic radius, ionization energy, and chemical reactivity.
Period: A Horizontal Journey through Energy Levels
Periods represent horizontal rows in the periodic table. They reflect the number of energy levels, or electron shells, that an atom has. The electrons in these energy levels determine an element’s chemical properties.
Group: A Vertical Exploration of Reactivity
Vertical columns in the periodic table are known as groups. Elements in a group have similar valence electrons, which are the electrons in the outermost energy level. The number of valence electrons influences chemical bonding and reactivity.
Valence Electrons: The Key to Chemical Bonding
Valence electrons are the electrons that participate in chemical bonding. They determine an element’s ability to form bonds with other atoms and its chemical properties.
Noble Gas Configuration: The Epitome of Stability
Noble gases have a stable electron configuration with a full outermost energy level. This gives them low reactivity and makes them ideal for use in various applications.
Introduce the concept of isotopes, mass spectroscopy, and elemental analysis.
Atomic Mass: The Sum of the Building Blocks
In the realm of chemistry, understanding the atomic mass of elements is crucial. This fundamental property represents the total mass of protons and neutrons found within the nucleus of an atom. Protons, positively charged particles, contribute significantly to the mass of an atom. Neutrons, on the other hand, have no charge but still add to its overall weight. By understanding the atomic mass, scientists can gain insights into the composition and behavior of various elements.
One intriguing concept related to atomic mass is the existence of isotopes. Isotopes are different forms of an element that share the same atomic number but vary in their number of neutrons. As a result, isotopes have identical chemical properties but distinct masses. By studying isotopes, scientists can investigate variations within elements and gain valuable information about atomic structure and radioactive decay.
Mass Spectroscopy and Elemental Analysis
Scientists utilize sophisticated techniques to determine the atomic mass of elements. Mass spectroscopy is a powerful tool that allows for the precise measurement of the mass-to-charge ratio of ions. This information can be used to identify and characterize different isotopes of an element. Through mass spectroscopy, scientists can gain insights into the isotopic composition of materials and trace the origins of elements in environmental or historical samples.
In addition to mass spectroscopy, elemental analysis is another important technique in chemistry. Elemental analysis provides qualitative and quantitative information about the elemental composition of a sample. By utilizing various methods such as atomic absorption spectroscopy, inductively coupled plasma mass spectroscopy, and X-ray fluorescence spectroscopy, scientists can determine the presence and concentration of specific elements in a substance. These analytical techniques play a crucial role in fields ranging from environmental monitoring to medical diagnostics and forensic science.
Unraveling the Essence of Matter: A Guided Tour Through the Periodic Table
In the realm of science, the periodic table stands as a beacon, illuminating the fundamental nature of matter. It’s a tapestry woven with the threads of elements, each unique in its essence and essential for life as we know it. Embark with us on a storytelling journey to unravel the secrets enshrined within this enigmatic chart.
Periodic Trends: A Symphony of Predictable Changes
Atomic number, the fundamental property that defines an element, acts as a maestro, orchestrating a predictable dance of chemical properties. As we journey across the periodic table, from left to right, the atomic number increases, unlocking a symphony of changes in chemical behavior.
These periodic trends manifest in myriad ways. The atomic radius, a measure of an atom’s size, shrinks as we move across a period, or horizontal row. This is because the increased nuclear charge draws the electrons closer to the nucleus. Conversely, moving down a group, or vertical column, the atomic radius increases, as additional energy levels accommodate more electrons.
Ionization energy, the energy required to remove an electron from an atom, also follows a predictable trend. It generally increases across a period and decreases down a group. This is due to the interplay between the nuclear charge and the number of electrons, which determines the strength of the electrostatic attraction.
Electron Configuration: The Blueprint of Atomic Identity
Electron configuration describes the arrangement of electrons in an atom’s energy levels. It plays a pivotal role in shaping an element’s chemical properties. Elements with similar electron configurations tend to exhibit similar chemical behavior. For instance, the noble gases, with their stable electron arrangements, are remarkably unreactive.
Valence Electrons: The Architects of Chemical Bonding
Valence electrons, the electrons in the outermost energy level, hold the key to chemical bonding. They determine an element’s ability to form bonds with other atoms, paving the way for the formation of molecules and the rich tapestry of chemical compounds. Elements with the same number of valence electrons often share similar bonding characteristics.
Noble Gas Configuration: The Epitome of Stability
Noble gas configuration represents the ultimate state of electron stability. When an atom achieves this configuration, it attains an inert state, with no tendency to react with other elements. The noble gases exemplify this stability, hence their designation as the “inert gases.”
The Periodic Table: A Legacy of Organization
The periodic table is not merely a catalog of elements; it’s a masterpiece of organization that embodies the principles of chemistry. It allows us to predict the properties of elements and understand their behavior. From Mendeleev’s pioneering work to the modern periodic law, the periodic table has guided our understanding of the atomic world.
The periodic table is an indispensable tool for unraveling the mysteries of matter. By understanding the concepts of atomic number, atomic mass, and periodic trends, we can appreciate the intricate dance of elements. The electron configuration, valence electrons, and noble gas configuration provide further insights into the chemical properties that shape our world. May this storytelling journey inspire you to explore the depths of chemistry and unravel the secrets of the periodic table, one element at a time.
Unraveling the Secrets of Elements: A Journey through Atomic Structure
In the realm of chemistry, understanding the nature of elements is fundamental. The atomic number unlocks the essence of an element, determining its identity and distinguishing it from others. It represents the number of protons, positively charged particles, in an atom’s nucleus.
Atomic mass, the sum of proton and neutron masses, provides insights into an element’s weight. The presence of isotopes, variants with varying neutron counts, reveals the complexity within elements. Mass spectrometry and elemental analysis techniques enable us to unravel these isotopic secrets.
Unveiling Patterns in the Elements: The Symphony of the Periodic Table
As scientists delved deeper into the world of elements, they discovered remarkable patterns. The periodic table emerged as a masterpiece, an organized arrangement revealing commonalities between elements. Periods, horizontal rows, showcase elements with progressively increasing atomic numbers. Each row represents a higher energy level, filled with electrons orbiting the nucleus.
Groups, vertical columns, group elements with similar outermost energy levels, known as valence electrons. These electrons play a pivotal role in determining an element’s chemical behavior and reactivity. Chemical properties, such as atomic radius and ionization energy, exhibit predictable trends across periods and groups, highlighting the influence of atomic structure on element behavior.
Chemical Bonding: The Dance of Valence Electrons
Valence electrons, like eager dancers, hold the key to chemical bonding. These electrons, residing in the outermost energy level, determine an element’s bonding potential. They participate in electron dot diagrams, visual representations of chemical bonding, showcasing the distribution of valence electrons and predicting molecular geometry.
The noble gas configuration, a stable arrangement of valence electrons resembling that of noble gases, provides insights into stability. Elements strive to attain this configuration, leading to chemical reactions that form stable compounds.
Thus, the atomic structure of elements, with its intricate interplay of protons, neutrons, and electrons, governs their properties, reactivity, and the remarkable tapestry of chemical substances that shape our world.
Describe periods as horizontal rows in the periodic table.
Decoding the Elements: Unraveling the Secrets of the Periodic Table
Our world is composed of an astounding array of elements, each possessing unique characteristics that drive the chemistry of life. Understanding the principles that govern these elements is crucial for deciphering the secrets of the natural world. Enter the periodic table, a remarkable tool that organizes elements based on their atomic number, a fundamental property that defines an element’s identity.
Understanding the Periodic Table
Imagine the periodic table as a map, with periods running horizontally and groups vertically. Each period represents a different energy level, which houses electrons in specific orbitals. The arrangement of electrons determines an element’s chemical properties, such as its reactivity and bonding behavior.
Periods: A Journey Through Energy Levels
Periods are horizontal rows in the periodic table that correspond to the principal energy level of an element’s electrons. As you move from left to right across a period, electrons fill the orbitals of the same energy level.
For instance, in the first period, the hydrogen atom has one electron occupying the first energy level. Helium, its right-hand neighbor, has two electrons filling the first energy level. This gradual filling of orbitals determines the unique properties of each element.
Groups: Exploring Reactivity
Groups are vertical columns in the periodic table that represent elements with the same number of valence electrons. Valence electrons are those in the outermost energy level and play a critical role in determining an element’s chemical bonding and reactivity.
Elements in the same group exhibit similar chemical properties due to their identical valence electron configuration. For example, the alkali metals in Group 1 have one valence electron, which makes them highly reactive and likely to lose electrons. The noble gases in Group 18, on the other hand, have a full complement of valence electrons, making them chemically inert.
Understanding periods and groups is essential for grasping the systematic organization of elements in the periodic table. This powerful tool provides a framework for comprehending the diversity of elements and their interplay in the world around us.
Delving into the Realm of Electron Configuration: Unraveling the Energy Levels and Atomic Orbitals
In the intricate tapestry of atomic structure, electron configuration emerges as a fundamental concept, shaping the chemical properties of elements and unveiling the enigmatic dance of electrons. It refers to the distribution of electrons within an atom’s energy levels and atomic orbitals, providing a glimpse into the quantum world of electrons.
Energy levels, aptly named, are discrete regions surrounding an atom’s nucleus where electrons reside. Each energy level is further divided into sublevels, denoted by letters (s, p, d, f). The s sublevel can accommodate up to two electrons, while the p sublevel holds a maximum of six. The d sublevel can host up to ten electrons, and the f sublevel can accommodate up to fourteen.
Within these sublevels lies the concept of atomic orbitals, which are three-dimensional regions in space where there is a high probability of finding an electron. Each sublevel corresponds to a specific type of orbital: s orbitals are spherical, p orbitals are dumbbell-shaped, d orbitals exhibit a more complex geometry, and f orbitals are even more intricately shaped.
As we venture into the realm of electron configuration, we encounter an underlying principle known as the Aufbau principle. It dictates that electrons fill up orbitals in a sequential manner, starting with the lowest energy level and sublevel. This meticulous filling pattern ensures that atoms adopt their most stable configurations, striving for minimal energy.
Understanding electron configuration is a crucial step in comprehending the chemical behavior of elements. It governs the formation of chemical bonds, determines oxidation states, and influences a myriad of chemical and physical properties. By deciphering the arrangement of electrons within atoms, we unlock a deeper understanding of the fundamental building blocks of our universe.
Define groups as vertical columns in the periodic table.
Unlocking the Secrets of the Periodic Table: A Journey through the Building Blocks of Matter
In the vast tapestry of the universe, the periodic table stands as a masterpiece, a roadmap to the fundamental elements that shape our world. Embark on a journey to unravel its secrets, uncovering the profound significance of atomic numbers and masses, periodic trends, and the captivating interplay of electrons.
Atomic Number: The Foundation of Elemental Identity
The heart of an atom lies in its nucleus, the abode of protons and neutrons. The atomic number emerges as a crucial parameter, representing the number of protons within this compact core. It is this number that defines an element, distinguishing it from all others. Imagine a bustling city where each building represents an atom; the number of doors in each building marks its atomic number.
Atomic Mass: The Sum of the Building Blocks
The mass of an atom, a collective weight of its protons and neutrons, emerges as the atomic mass. Isotopes, variations of an element with differing neutron counts, provide a fascinating glimpse into the atomic realm. Like twins with slightly different appearances, isotopes share the same atomic number but possess distinct atomic masses.
Periodic Trends: Uncovering the Properties’ Patterns
As we journey across the periodic table, a remarkable dance of properties emerges. Periodic trends reveal predictable changes in chemical properties based on atomic number. Picture a shimmering rainbow, where each color represents a specific property; the horizontal progression across the table mirrors the gradual shift in these hues.
Period: A Horizontal Journey through Energy Levels
Periods, the horizontal rows in the periodic table, unveil the tale of electron placement. Each row corresponds to an energy level, where electrons gracefully orbit the nucleus. Like dancers on a stage, electrons occupy specific energy levels, dictating the atom’s chemical behavior.
Group: A Vertical Exploration of Reactivity
Descending the vertical columns, known as groups, unveils a different perspective. Electrons in the outermost energy level, the valence electrons, play a pivotal role in chemical bonding and reactivity. It’s like a team of explorers venturing into uncharted territories, ready to forge connections and shape the atomic landscape.
Group: A Vertical Exploration of Reactivity
In the tapestry of the periodic table, groups emerge as vertical columns that unveil the secrets of reactivity. Each group harbors elements with a common characteristic: their valence electrons. These electrons, residing in the outermost energy level, play a pivotal role in chemical bonding and reactivity.
Valence electrons determine an element’s ability to form bonds with other atoms. Elements within a group possess a consistent number of valence electrons, endowing them with similar chemical behaviors. This kinship stems from the fact that the valence electrons are the ones that participate in bonding.
Reactivity refers to an element’s tendency to engage in chemical reactions. It is directly influenced by the number of valence electrons. Elements with fewer valence electrons are more reactive because they require fewer bonds to attain a stable electron configuration, resembling that of noble gases. Conversely, those with a high number of valence electrons are less reactive.
By delving into the vertical columns of the periodic table, we uncover a fascinating narrative of reactivity and chemical bonding. Groups provide a roadmap to understanding how elements interact with each other and pave the way for exploring endless possibilities in the world of chemistry.
The Chemistry of Elements: Unraveling the Secrets of Matter
Introduction: In the realm of science, the periodic table stands as a testament to the intricate organization of the universe. This indispensable tool unveils the mysteries of the elements, unraveling the secrets that govern their behavior and interactions. Join us as we embark on a captivating journey through the fundamental building blocks of our world.
Atomic Number: The Heart of Identity
At the heart of every element lies its atomic number, a defining characteristic that sets it apart from all others. This number, representing the number of protons in an atom’s nucleus, serves as the very foundation of an element’s identity. Protons, along with neutrons, collectively known as nucleons, reside in the nucleus, the tiny, dense core of the atom.
Atomic Mass: The Weight of the Building Blocks
The weight of an atom, its atomic mass, arises from the combined masses of its protons and neutrons. This mass determines an element’s physical properties, influencing its density, boiling point, and melting point. The concept of isotopes, atoms with the same atomic number but varying numbers of neutrons, sheds light on the variability within elements. Mass spectroscopy and elemental analysis provide valuable tools for identifying and characterizing these isotopic variations.
Periodic Trends: Predicting the Future
As we progress through the periodic table, a remarkable pattern emerges. Chemical properties, like reactivity and electronegativity, change in a predictable manner as we move across rows and down columns. These periodic trends offer a glimpse into the future behavior of elements based on their position in the table. Atomic radius, ionization energy, and electron affinity are but a few of the properties that follow these well-defined patterns.
Periods: A Horizontal Journey through Energy Levels
Periods, the horizontal rows of the periodic table, take us on a journey through the energy levels of an atom. Each period introduces a new energy level, with electrons occupying the lowest energy levels first. This arrangement lays the foundation for the chemical properties that distinguish each element.
Groups: A Vertical Exploration of Reactivity
Venturing down the vertical columns of the periodic table, known as groups, we delve into the world of valence electrons, those electrons residing in the outermost energy level. These electrons play a crucial role in chemical bonding, determining an element’s reactivity and bonding characteristics. The number of valence electrons directly influences an element’s placement within a group.
The Alchemy of Bonding: Exploring the Role of Valence Electrons
In the tapestry of chemistry, valence electrons stand as the pivotal players in the intricate dance of chemical bonding. These electrons, residing in the outermost energy level of atoms, hold the key to understanding the chemical behavior and properties of elements.
Imagine valence electrons as the architects of bonding, the glue that holds atoms together to form molecules. They participate in the mesmerizing process of chemical bonding, where atoms share or transfer electrons to achieve a stable configuration. This delicate exchange determines the properties of the resulting compounds, shaping the world around us.
The number of valence electrons an atom possesses directly influences its oxidation state, which represents the ability of the atom to donate or accept electrons. This characteristic is crucial in understanding the reactivity and bonding tendencies of elements.
Moreover, valence electrons unveil the secrets of electron dot diagrams. These visual representations depict the valence electron arrangement of atoms, providing insights into their bonding behavior. By arranging dots around the atomic symbol, scientists can predict molecular geometry and bond types.
In essence, valence electrons orchestrate the symphony of chemical bonding. Their dynamic nature and influence on oxidation states and electron dot diagrams lay the foundation for understanding the intricate relationships between elements and the properties of matter.
Define noble gas configuration as an electron arrangement resembling that of noble gases.
Journey into the Heart of Elements: Unraveling the Secrets of Atomic Structure
In the vast tapestry of the universe, elements play a pivotal role in shaping the world around us. Understanding their fundamental properties is key to unlocking the mysteries of chemistry and materials science.
The Quintessence of Identity: Atomic Number
Each element is defined by its unique atomic number, which represents the number of protons in its nucleus. These positively charged protons form the core of the atom and determine its identity. Nucleons, the collective term for protons and neutrons, contribute to the atomic mass.
Atomic Mass: The Sum of Its Parts
Atomic mass represents the combined mass of protons and neutrons within an atom. Variations in neutron number give rise to different isotopes of the same element. Mass spectrometry and elemental analysis techniques allow us to determine the isotopic composition of elements.
Periodic Trends: Patterns in the Elements’ Behavior
As we traverse the periodic table, we observe predictable changes in chemical properties known as periodic trends. These trends can be explained by the variation in atomic number and the resulting electronic structure.
Period: Energy Levels Unraveled
Periods are horizontal rows in the periodic table that represent atoms with the same energy level configuration. Electron configuration refers to the arrangement of electrons in different energy levels.
Group: Reactivity Explored
Groups, vertical columns in the table, bring together elements with similar valence electrons. Valence electrons play a crucial role in chemical bonding and determine an element’s reactivity.
Valence Electrons: The Gateway to Bonding
Valence electrons reside in the outermost energy level and govern an element’s oxidation states and participation in chemical reactions.
Noble Gas Configuration: Inert and Stable
Noble gas configuration describes an electron arrangement that mirrors that of noble gases, the most stable elements. This stable configuration explains the inert nature of noble gases and their adherence to the octet rule.
Electron Dot Diagram: Visualizing Chemical Bonding
Electron dot diagrams depict valence electrons as dots and provide a handy tool for predicting molecular geometry and understanding chemical bonding.
Noble Gas Configuration: The Epitome of Stability
Imagine a world of perfect balance and stability, where everything is in its rightful place. This is the world of noble gas configuration.
Noble gases, like helium and argon, are the epitome of this stability because of their unique electron arrangement. Their outermost energy level is filled with electrons, giving them an electron configuration similar to that of helium, the first noble gas. This configuration grants them an unparalleled inertness, making them the least reactive elements in the periodic table.
The octet rule is a fundamental principle in chemistry that explains why noble gases are so stable. This rule states that atoms tend to form bonds and rearrange their electrons until they achieve an electron configuration similar to that of a noble gas, where their outermost energy level is filled with eight electrons.
Stability implies a reluctance to change. Noble gases, with their complete outermost energy levels, have no desire to form bonds with other atoms. They are content in their solitary existence, floating through the world of atoms without any chemical drama.
The noble gas configuration serves as a testament to the fundamental nature of the universe. It shows us that stability is a desirable state and that atoms strive to achieve it through interactions with each other. It is a reminder that balance and harmony are essential for the smooth functioning of any system.
Understanding Atomic Structure and the Building Blocks of Matter
The world around us is made up of countless atoms, the fundamental building blocks of all matter. Each atom is unique, with its own set of characteristics that define its identity and behavior. Two of the most important properties of atoms are atomic number and atomic mass, and learning about them is like embarking on a thrilling journey into the heart of matter.
Atomic number is the number of protons in an atom’s nucleus, the central part of the atom. Protons have a positive charge, and their number determines which element an atom belongs to. For example, all atoms with an atomic number of 6 are carbon atoms.
Atomic mass, on the other hand, is the sum of the masses of the atom’s protons and neutrons, which are also found in the nucleus. Neutrons, as their name suggests, have no charge. The number of neutrons can vary among atoms of the same element, resulting in different isotopes of that element.
These two properties, atomic number and atomic mass, are crucial for understanding the behavior of elements. They play a key role in determining an element’s chemical properties and its place in the periodic table, a powerful tool that organizes elements based on their similarities.
So, as we delve deeper into the realm of atomic structure, remember that atomic number and atomic mass are the fundamental building blocks that shape the world of matter, one atom at a time.
Discuss their use in Lewis structures, chemical bonding, and predicting molecular geometry.
The Journey Through the Periodic Table: Unraveling the Secrets of Elements
Imagine embarking on a grand adventure, exploring the intricacies of the universe that surrounds us. Our first stop is the periodic table, a roadmap guiding us through the vast realm of elements. Let’s decode the secrets hidden within its rows and columns.
Atomic Number and Mass: The Defining Characteristics
At the heart of every element lies its atomic number, which is the number of protons residing in its nucleus. Like a fingerprint, this number uniquely identifies each element, distinguishing it from all others. Closely linked to the atomic number is the atomic mass, the sum of protons and neutrons within an atom’s nucleus.
Periodic Trends: Uncovering Hidden Patterns
As we journey across the periodic table, we discover fascinating patterns in the elements’ properties, known as periodic trends. These trends arise from the predictable changes in atomic number and electron configuration. For instance, atomic radius generally decreases moving from left to right across periods, while ionization energy increases.
Periods and Groups: A Structured Exploration
The periodic table is organized into periods (horizontal rows) and groups (vertical columns). Each period represents a new energy level, while groups share a similar number of valence electrons. Valence electrons are the key players in chemical bonding, the process by which atoms join together to form molecules.
Noble Gas Configuration: The Pinnacle of Stability
Certain elements, known as noble gases, possess a stable electron configuration resembling that of noble gases. This configuration, with a full outermost energy level, renders these elements highly unreactive. Understanding this configuration is essential for comprehending the chemical behavior of elements.
Electron Dot Diagrams: A Visual Guide to Bonding
Electron dot diagrams, like tiny molecular maps, depict valence electrons as dots surrounding the element symbol. These diagrams provide valuable insights into chemical bonding. By sharing or transferring valence electrons, atoms achieve a more stable configuration, leading to the formation of molecules with specific shapes and properties.
The Periodic Table: A Timeless Masterpiece
The periodic table, conceived by Mendeleev, is a testament to the power of human ingenuity. It serves as a universal language for chemists, unifying our understanding of the elements and their myriad properties. By mastering this framework, we unlock the gateway to unraveling the mysteries of chemistry and beyond.
The Periodic Table: A Journey Through the Heart of Chemistry
Imagine a world where every building block of matter is arranged in a magnificent tapestry, each element occupying its rightful place in a symphony of organization. This is the realm of the Periodic Table, a masterpiece of science that unravels the secrets of the universe, one atom at a time.
At the core of this intricate framework lies the atomic number, a fundamental identifier that defines each element’s unique identity. It represents the number of protons, the positively charged particles within the atom’s nucleus. These microscopic building blocks determine the element’s specific characteristics, paving the way for its distinct properties and behavior.
Accompanying the protons are neutrons, electrically neutral particles that contribute to the atom’s overall mass. The sum of the proton and neutron counts gives rise to the atomic mass, a measure of the atom’s heft. This subtle interplay between protons and neutrons governs the atom’s stability and its role in the wider chemical landscape.
As we embark on a horizontal journey through the periodic table, we encounter periods, rows of elements with an increasing number of electrons. These negatively charged particles orbit the nucleus, occupying specific energy levels. The arrangement of electrons in these energy levels, known as electron configuration, dictates an element’s chemical properties, including its reactivity and ability to form bonds.
Descending vertically through the table, we explore groups, columns of elements with similar chemical properties. These elements share a common number of valence electrons, the electrons in the outermost energy level. Valence electrons play a pivotal role in chemical bonding, determining the type and strength of interactions between atoms.
By understanding the principles that govern the periodic table, we unlock the secrets of chemistry. It empowers us to predict the reactivity of elements, design new materials, and unravel the complexities of the natural world. From the formation of water molecules to the synthesis of life-saving drugs, the periodic table is an indispensable tool that guides our journey through the molecular realm.
In its essence, the periodic table is a testament to the interconnectedness of the universe, a symphony of elements whose properties and interactions shape the fabric of our existence. It is a masterpiece of science that continues to inspire and empower generations of explorers, unlocking the secrets of the cosmos, one atom at a time.
Unraveling the Secrets of the Chemical Cosmos: A Journey through the Periodic Table
In the realm of chemistry, the periodic table stands as an enigmatic masterpiece, revealing the fundamental patterns that govern the behavior of the elements. Its origins can be traced back to the pioneering work of Dmitri Mendeleev in the 1860s, who arranged the elements in a tabular format based on their atomic weights and chemical properties.
Mendeleev’s revolutionary approach unveiled striking periodic trends – predictable changes in chemical properties that occur as we move across and down the table. These trends, including atomic radius, ionization energy, and electronegativity, reflect the underlying electronic structures of the elements.
The periodic table’s organization into periods (horizontal rows) and groups (vertical columns) provides a wealth of insights. Periods delve into the electron configuration and energy levels of atoms, while groups highlight valence electrons, which determine an element’s chemical bonding and reactivity.
The pursuit of understanding the periodic table has led to the discovery of isotopes – atoms of the same element with varying neutron numbers. This intricate interplay of protons, neutrons, and electrons governs the unique identities and properties of each element.
The noble gas configuration holds a special significance in the periodic table, symbolizing stability and inertness. Elements with this configuration, such as helium and neon, have a complete outer shell of electrons, rendering them less reactive than their counterparts.
To visualize chemical bonding, scientists have devised electron dot diagrams, which represent valence electrons as dots. These diagrams aid in predicting molecular geometry and understanding the chemical interactions between atoms.
The periodic table is an invaluable tool for chemists, providing a comprehensive reference for element properties, reactivity, and bonding behavior. Its significance extends beyond the laboratory, influencing fields as diverse as medicine, materials science, and environmental studies.
As we continue to explore the intricacies of the periodic table, new discoveries await, promising to deepen our understanding of the fundamental building blocks of our universe.