The Periodic Table: A Comprehensive Guide To Element Organization
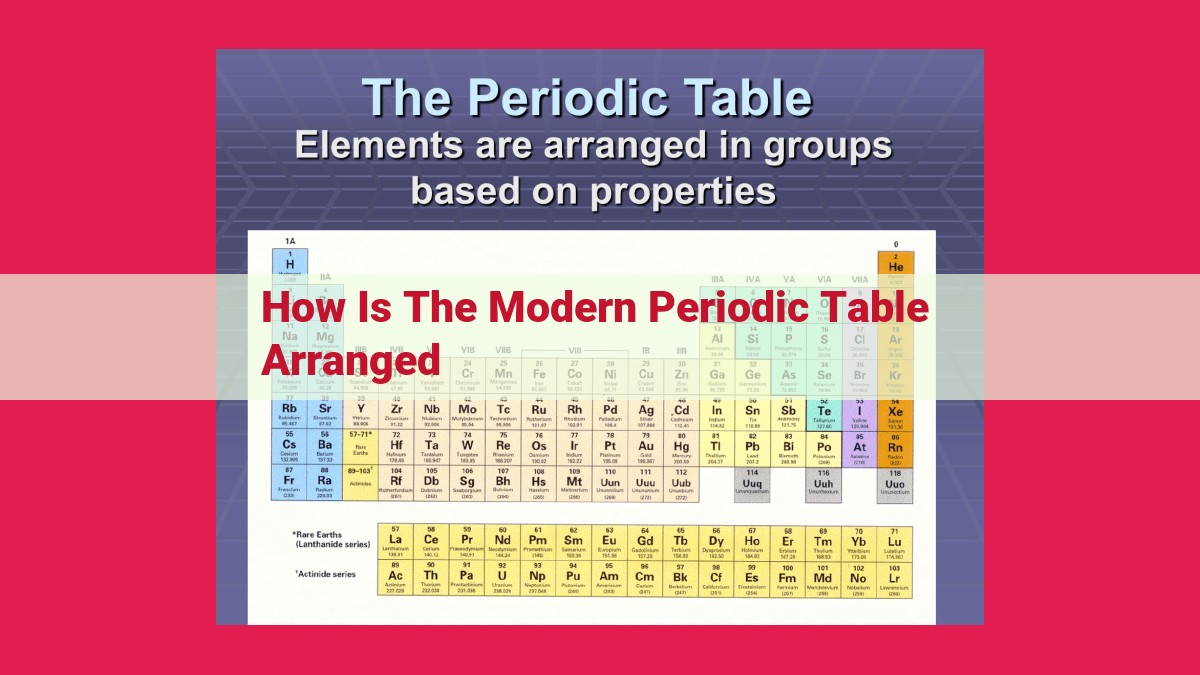
The modern periodic table organizes elements in ascending order of atomic number, allowing for the prediction of their properties and behavior. Elements are grouped based on similarities in their chemical properties, resulting in vertical columns known as groups. Across horizontal rows, called periods, elements share the same number of electron energy levels. The table further classifies elements as metals, nonmetals, or metalloids based on their properties, with noble gases being the most stable and inert. Understanding this arrangement enables scientists to predict the reactivity, ionization energy, and other characteristics of elements.
The Periodic Table: A Guide to the Elements
Atomic Number and Atomic Mass: The Building Blocks of Atoms
Every element in the periodic table is unique, defined by its atomic number and atomic mass. The atomic number, represented by the symbol Z, indicates the number of protons in an atom’s nucleus. Protons carry a positive charge and contribute significantly to the atom’s mass.
The atomic mass, symbolized as A, represents the average mass of an atom of a given element. It takes into account the different isotopes of the element, which are variations of the same element with varying numbers of neutrons. Neutrons are found in the nucleus alongside protons but carry no electrical charge. The different isotopic compositions of an element influence its atomic mass.
Understanding the Arrangement of the Modern Periodic Table
The periodic table, an iconic chart that organizes the known elements of the universe, is a testament to the order and patterns found in nature. Understanding its arrangement is key to unlocking the secrets of matter itself.
Unveiling the Basics: Atomic Number and Atomic Mass
Every element in the periodic table is defined by two fundamental properties: its atomic number and atomic mass. The atomic number, represented by Z, denotes the number of protons, or positively charged particles, in an atom’s nucleus. It’s what uniquely identifies an element and determines its position in the table.
The atomic mass, on the other hand, represents the average mass of an atom of the element. This value is influenced by the number of protons and neutrons (uncharged particles) in the nucleus. Atoms of the same element can have different numbers of neutrons, giving rise to isotopes. Isotopes have the same atomic number but varying atomic masses. This variation in neutron count contributes to the fractional values often seen in atomic masses.
Embracing the Periodic Law and Beyond
The periodic law, first proposed by Dmitri Mendeleev in the 19th century, serves as the cornerstone of the periodic table. This law states that elements arranged in order of increasing atomic number exhibit a repeating pattern of chemical and physical properties.
The periodic table is organized into horizontal rows called periods, and vertical columns called groups. Elements within the same group share similar chemical properties because they have the same number of valence electrons, the electrons that participate in chemical reactions. Conversely, elements within the same period share similar physical properties, such as atomic size and ionization energy, due to having the same number of energy levels.
Arrangement of the Modern Periodic Table: A Tale of Organizing the Elements
In the vast expanse of the universe, where countless celestial bodies dance amidst the cosmic void, there exists a remarkable tapestry of elements that form the building blocks of our existence. To unravel this tapestry and understand the intricate relationships between these elements, we turn to the periodic table.
The periodic table is a systematic arrangement of the chemical elements, organized based on their atomic number and atomic mass. The atomic number, represented by the number of protons in an atom’s nucleus, determines an element’s identity. The atomic mass, a weighted average of the masses of an element’s isotopes, provides insights into its nuclear composition.
The cornerstone of the periodic table is the periodic law, which states that the properties of elements repeat in a predictable manner as their atomic numbers increase. This law forms the foundation for organizing elements into the table’s rows and columns. The horizontal rows are called periods, and they represent elements with the same number of energy levels. The vertical columns are called groups, and they contain elements with similar chemical properties.
By studying the arrangement of elements in the periodic table, we can glean valuable information about their behavior and reactivity. Metals, located on the left side of the table, tend to be shiny, malleable, and good conductors of electricity. Nonmetals, found on the right side, are typically dull, brittle, and poor conductors. Metalloids, which occupy the border between metals and nonmetals, exhibit a blend of properties from both groups.
The group number of an element indicates the number of valence electrons it possesses. Valence electrons are the outermost electrons of an atom, which largely determine its chemical reactivity. Elements in the same group share similar valence electron configurations, giving them analogous chemical properties.
Periods, on the other hand, reflect the number of energy levels in an atom. As we move from left to right within a period, the elements gain protons and electrons, resulting in an increase in atomic number and ionization energy. Ionization energy is the energy required to remove an electron from an atom, and it generally increases across a period.
The noble gases, located in Group 18, are a unique set of elements that stand apart from the rest. They are highly stable and unreactive due to their full valence shells, making them ideal for various technological applications.
Understanding the arrangement of elements in the periodic table empowers us to predict their behavior, unravel chemical reactions, and explore the vast realm of chemistry. It is a testament to the human pursuit of knowledge, a roadmap that guides us through the intricacies of the atomic world.
The Orderly Universe of the Periodic Table
In the realm of chemistry, the periodic table is a roadmap that guides our understanding of the elements that make up our world. It’s a captivating story of patterns, predictions, and the dance of electrons that define each element’s unique personality.
As we traverse the table, we encounter groups of elements lined up vertically, like families of siblings. These groups share a common trait: the number of valence electrons, those electrons in the outermost energy level that determine an element’s chemistry. Like people with similar interests, group members tend to have схожие chemical behaviors.
Horizontally, we have periods, rows of elements that ascend as we add electrons. Each period represents a new energy level, like rungs on a ladder. As we move across a period, the atomic number (the number of protons in an atom) increases, shaping the element’s identity and properties.
The periodic table unveils a remarkable choreography of ionization energy, the dance of electron removal. As we venture from left to right across a period, ionization energy rises. This means it becomes harder to pull away electrons, hinting at the element’s unwillingness to let go of its outermost electrons.
Conversely, as we descend a group, ionization energy decreases, making electrons more eager to escape. This behavior reflects the growing number of energy levels, providing ample space for electrons to roam freely.
So, as we wander through the periodic table, we marvel at how the grouping of elements unveils their chemical kinship and the rise and fall of periods narrates the waltz of electrons. Each element, with its unique set of properties and behaviors, emerges from this symphony of atoms, enriching our understanding of the chemical universe.
Unveiling the Secrets of the Periodic Table: Classifying Elements as Metals, Nonmetals, and Metalloids
Ever wondered how chemists organize the plethora of elements in the universe? It’s not just a random arrangement; it’s a meticulously designed system called the periodic table. And one of its key concepts is the classification of elements into three distinct categories: metals, nonmetals, and metalloids.
Metals:
Imagine a group of elements that are shiny, malleable (you can bend them easily), and eager to form a bond with other atoms. These are your metals. Think of your favorite utensils, cookware, and even jewelry—they’re likely made from metals like iron, copper, or gold. Metals are also excellent conductors of heat and electricity, making them essential in everything from power lines to electronic devices.
Nonmetals:
On the opposite end of the spectrum, we have nonmetals. They’re dull in appearance, brittle (snap easily), and generally not so keen on sharing their atoms. Sulfur, oxygen, and chlorine are examples of nonmetals. Interestingly, they tend to be insulators, meaning they don’t conduct heat or electricity well. But that’s not all; many nonmetals are highly reactive, forming compounds with other elements.
Metalloids:
Now, let’s talk about the fence-sitters—the metalloids, a fascinating group of elements that share characteristics of both metals and nonmetals. They have an intermediate appearance, malleable, yet somewhat brittle. Boron, silicon, and germanium fall under this category. Metalloids can be both conductors and insulators depending on the conditions, and they’re often used in electronic devices due to their unique properties.
So, there you have it—the three main classifications of elements in the periodic table. Metals, nonmetals, and metalloids each bring their own unique traits to the scientific world. From the shiny, conductive metals we use in everyday life to the reactive nonmetals that form the air we breathe and the metalloids that bridge the gap between the two, these elements play a crucial role in shaping our world.
The Arrangement of the Modern Periodic Table: Unveiling the Secrets of Elements
Atomic Number and Atomic Mass: The Building Blocks of Matter
Every element in the periodic table is defined by its unique atomic number, the number of protons in its nucleus. Protons give an atom its identity. Atomic mass, on the other hand, is the average mass of its atoms, considering the different numbers of neutrons in its isotopes. Isotopes are variations of an element with varying neutron counts, contributing to the mass variations within an element.
Periodic Law and Trends: Order and Patterns in the Elements
The periodic law governs the arrangement of elements in the table. It states that elements with similar chemical properties appear in vertical columns called groups. As you move down a group, the number of energy levels increases, giving the elements additional properties. Elements also align horizontally in periods, which have the same number of energy levels. The periodic table showcases the remarkable order and patterns in the element world.
Metal, Nonmetal, and Metalloid Classification: Diverse Properties, Distinct Roles
The periodic table divides elements into three main categories: metals, nonmetals, and metalloids. Metals are generally shiny, malleable, and good conductors of heat and electricity. Think of copper wires in your house or the steel in your car. Nonmetals, on the other hand, are often dull, brittle, and poor conductors. They form the backbone of molecules in many organic compounds. Metalloids possess characteristics of both metals and nonmetals, bridging the gap between these two extremes.
Groups and Ions: Connecting Reactivity to Electron Configuration
The groups in the periodic table are numbered 1-18 and represent elements with specific chemical properties. The number of valence electrons in an element’s outermost energy level determines its reactivity and ability to form ions. Elements in the same group share similar valence electron configurations, influencing their chemical behavior.
Periods and Ionization Energy: Unveiling Energy Barriers
Periods in the periodic table indicate the number of energy levels in an atom. As you move from left to right across a period, the elements gain protons and electrons. This results in an increase in ionization energy, the energy required to remove an electron from an atom. Ionization energy provides insights into the stability and reactivity of elements.
Noble Gases: The Inert Giants at the Edge
Noble gases occupy Group 18 and are characterized by their low reactivity and stability. This is attributed to their filled valence shells, which make them chemically inert. Noble gases play vital roles in various applications, from lighting (neon) to medical imaging (helium).
By understanding the arrangement of the periodic table, we gain insights into the fundamental properties and behaviors of elements. This knowledge forms the cornerstone of chemistry and enables us to harness the power of elements for countless applications that shape our modern world.
The Significance of Groups in the Periodic Table
Imagine the periodic table as a bustling city, with rows of buildings representing the periods and columns of buildings representing the groups. Each group is a neighborhood with elements sharing similar characteristics, just like residents of a neighborhood share traits and backgrounds.
The groups are numbered 1 to 18 from left to right. These numbers are not just arbitrary labels but carry vital information about the elements’ properties. The group number of an element reveals its valence electron count, which plays a crucial role in determining its chemical behavior.
For instance, elements in Group 1 have one valence electron, making them highly reactive. They readily give up this electron to achieve stability and easily form positive ions. In contrast, elements in Group 17 have seven valence electrons, making them relatively inert and more likely to form negative ions.
The group number also indicates the element’s outermost energy level. For example, all elements in Period 2 have their valence electrons in the second energy level. This shared feature leads to similar chemical properties within the period.
Understanding the significance of groups helps us comprehend the chemical identity of elements. By knowing the group number, we can predict their reactivity, ionic tendencies, and even their physical characteristics like appearance, conductivity, and malleability. This knowledge empowers us to understand how elements interact and form the basis of chemistry.
Unlocking the Secrets of the Modern Periodic Table
Metal, Nonmetal, and Metalloid Classification
From shiny gold to brittle oxygen, the world is adorned with an array of elements, each with its own unique set of properties. The modern periodic table provides a systematic arrangement of these elements, revealing patterns that unlock their secrets.
The Significance of Groups
Elements in the periodic table are grouped into columns, known as groups, numbered from 1 to 18. Each group shares similar chemical properties due to the number of valence electrons they possess. Valence electrons are the electrons found in the outermost energy level of an atom, and they determine an element’s reactivity and ability to form ions.
Valence Electrons and Reactivity
The number of valence electrons plays a crucial role in determining an element’s reactivity. Elements with fewer valence electrons tend to be more reactive because they readily form bonds with other atoms to achieve a stable octet of valence electrons. In contrast, elements with more valence electrons are less reactive as they have a more stable electron configuration.
Ionization Energy
Ionization energy refers to the amount of energy required to remove an electron from an atom. As you move across a period (row) from left to right, the ionization energy generally increases. This is because the effective nuclear charge (the net positive charge of the nucleus) increases across the period, making it more difficult to remove an electron.
Noble Gases: The Stable Exception
Noble gases, found in Group 18, possess a complete octet of valence electrons, making them exceptionally stable and nonreactive. This stability stems from the fact that they have no need to gain or lose electrons to attain a stable configuration. Their low reactivity makes them highly valuable in applications such as lighting, welding, and medical imaging.
Understanding the groupings and valence electrons of elements is essential for deciphering their chemical behavior. The periodic table is a powerful tool that provides a roadmap to understanding the properties and reactions of elements, empowering us to unravel the mysteries of the atomic world.
Describe the arrangement of elements within periods, which have the same number of energy levels.
5. Periods and Ionization Energy
Within the periodic table, periods refer to the horizontal rows of elements, which represent the number of energy levels within their atoms. As you move from left to right across a period, the atomic number (number of protons) increases by one.
This stepwise increase in atomic number corresponds to an increase in the number of electrons in the outermost energy level, known as the valence shell. This, in turn, significantly influences the element’s chemical properties and reactivity.
Ionization energy, the energy required to remove an electron from an atom, also exhibits distinct trends across periods. In general, ionization energy increases as you move from left to right within a period. This is because the increasing number of electrons in the valence shell creates a stronger electrostatic attraction between the nucleus and the outermost electrons, making it more difficult to remove them.
Ionization Energy: Unveiling the Power to Remove an Electron
In the realm of atoms, ionization energy holds a crucial role in understanding how elements interact with their surroundings. It’s the energy required to remove an electron from an atom, a process that unveils the atom’s reactivity and behavior.
Picture this: an atom, with its nucleus of protons and neutrons, is surrounded by a cloud of electrons orbiting at different energy levels. Ionization energy is the exact amount of energy we need to overcome to pull one of these electrons away from the atom’s embrace.
Now, why does ionization energy matter? It’s like a key that unlocks the secrets of an element’s chemical properties. The more easily an electron can be removed, the lower the ionization energy, and the more reactive the element. This is because reactive elements have a strong tendency to form ions, atoms with an unbalanced number of electrons and protons.
For instance, sodium, with its low ionization energy, readily loses an electron, forming a positively charged sodium ion (Na+). On the other hand, noble gases, such as helium and argon, have high ionization energies, which explains their remarkable stability and low reactivity.
As we move across a period in the periodic table, ionization energy generally increases. This is because the number of protons in the nucleus increases, creating a stronger attraction for the electrons. Conversely, as we descend a group, ionization energy decreases, indicating that the electrons are further away from the nucleus and experience a weaker attraction.
Understanding ionization energy is essential for unraveling the mysteries of chemical reactions, bond formation, and various other phenomena. It’s the key to unlocking the secrets of how atoms interact with each other and the world around them.
The Modern Periodic Table: A Symphony of Elements
Prologue:
Journey with us through the captivating tapestry of the modern periodic table, an ingenious arrangement that unveils the secrets of the universe’s building blocks. Embark on an expedition to understand the order behind the chaos, the dance of electrons, and the symphony of elements.
Chapter 1: The Symphony’s Score: Atomic Number and Mass
Each element in this symphony bears a unique signature, its atomic number. This number, representing the number of protons, determines an element’s position in the table. Atomic mass, the average weight of an atom, holds clues to the number of neutrons, the enigmatic particles that add weight to the nucleus.
Chapter 2: The Periodic Rhythm: Like-Minded Elements Dance
The periodic table is not merely a random collection; it’s a meticulously orchestrated symphony. Groups, or vertical columns, gather elements that share similar chemical traits. These groups, numbered 1-18, are like choirs singing the same tune. Periods, or horizontal rows, unite elements with the same energy level.
Chapter 3: Elements’ Profiles: Metals, Nonmetals, and Metalloids
Within the periodic table’s melody, we encounter three distinct classes of elements: metals, with their shimmering appearance and electrical prowess; nonmetals, the shy dancers with low conductivity; and metalloids, the versatile bridge that connects the two.
Chapter 4: Groups and Ions: The Waltz of Reactivity
The groups within the periodic table hold immense significance. Valence electrons, the outermost electrons, determine an element’s reactivity. They orchestrate the formation of ions, charged atoms that eagerly seek balance.
Chapter 5: Periods and Ionization Energy: A Measure of Strength
As we move across periods, elements gain an extra energy level. This change significantly impacts their ionization energy, the energy required to pry an electron from an atom’s grip.
Chapter 6: Noble Gases: The Serenading Elite
Finally, we reach the pinnacle of stability: noble gases. Inert and aloof, these elements occupy Group 18, with their valence shells filled to perfection. Their reluctance to react earned them the name “noble,” adding a touch of elegance to the periodic table’s symphony.
Epilogue:
The periodic table is a testament to science’s ability to decipher nature’s intricate designs. By understanding the arrangement and properties of elements, we unlock the secrets of the universe. And like any symphony, the periodic table forever echoes the cosmic harmony that governs our world.
Unraveling the Mystery of Noble Gases: The Secret to Their Enigmatic Inactivity
Embark on an enchanting journey as we delve into the realm of the periodic table and uncover the secrets that lie within. Our focus today: the enigmatic noble gases, masters of stability and low reactivity.
These elusive elements reside in Group 18, a sanctuary of tranquility within the periodic table’s vibrant tapestry. Their defining characteristic? Filled valence shells. But what exactly does that mean and how does it render them so aloof from chemical reactions?
Imagine an atom as a miniature solar system, with electrons orbiting the nucleus like planets. The valence electrons are those orbiting the outermost energy level, and their number dictates an element’s reactivity. When an element has a filled valence shell, it means that all the available spots in its outermost energy level are occupied by electrons. This arrangement creates a stable configuration, much like a complete puzzle.
In contrast, elements with unfilled valence shells are constantly seeking to complete their electronic puzzle, making them prone to forming chemical bonds with other elements. But noble gases have no such cravings. Their valence shells are already full, leaving them with a sense of contentment. They lack the impetus to engage in chemical reactions, hence their low reactivity.
Think of noble gases as self-sufficient loners, perfectly happy in their own company. They have no need to bond with others, as they already possess the electronic harmony they crave. This explains their inert nature and their tendency to remain isolated in their Group 18 corner.
Their stability also explains why noble gases are often used in applications where chemical reactivity is undesirable. For instance, helium is used in balloons because it is non-reactive and won’t burst into flames. Argon is utilized in incandescent light bulbs to prevent the filament from oxidizing. And xenon finds its niche in medical imaging, providing a clear and non-toxic contrast agent.
So, the next time you encounter a noble gas, remember the secret to its enigmatic inactivity: filled valence shells. These elements have mastered the art of self-sufficiency, embracing stability and eluding the allure of chemical reactions.