Master The Art Of Calculating Percent Change In Mass
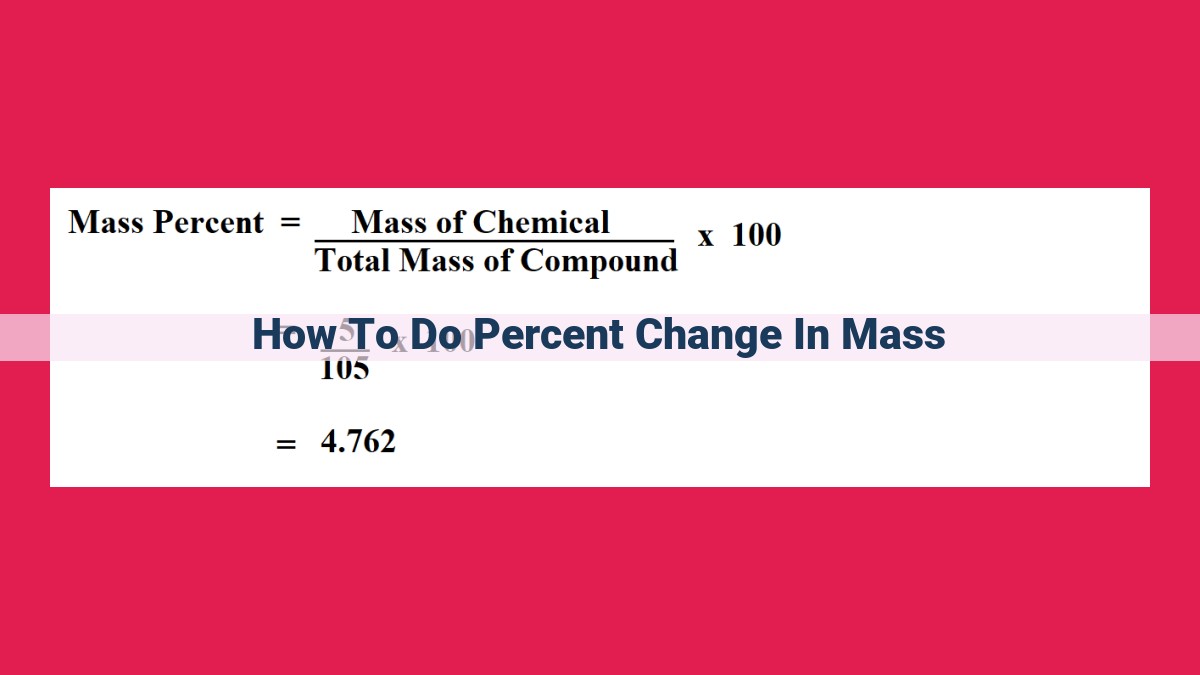
To calculate percent change in mass, determine the initial and final mass of the substance. Use the formula [(Final Mass – Initial Mass) / Initial Mass] x 100. Substitute the values and multiply the result by 100. This will give you the percentage change in mass. Remember to use consistent units and round answers appropriately. Consider examples like evaporation or chemical reactions where mass changes occur.
Understanding Mass and Weight: A Primer for Mass Calculations
In the realm of science, understanding the fundamental concepts of mass and weight is crucial for accurate calculations and a thorough comprehension of various physical processes. Let’s delve into the definitions and units of measurement associated with these two essential properties.
Mass: The Inherent Matter
Mass refers to the amount of matter present in an object. It remains constant regardless of the object’s location or the forces acting upon it. Mass is measured in units of grams (g) or kilograms (kg).
Weight: The Force of Gravity’s Embrace
Weight, on the other hand, is the force exerted on an object due to gravitational pull. It is dependent on both the mass of the object and the gravitational field strength in which it is located. The greater the mass or the stronger the gravitational field, the heavier the object. Weight is measured in units of newtons (N) or pounds (lbs).
Units of Measurement
For mass, we commonly use grams (g) and kilograms (kg). A gram is a small unit suitable for measuring the mass of lightweight objects, while a kilogram is larger, representing a thousand grams.
Conversions
When necessary, it’s essential to convert between mass units to ensure consistency in calculations. One kilogram (kg) is equivalent to 1,000 grams (g).
Initial and Final Mass: The Crucial Distinction
When measuring mass, it’s imperative to differentiate between its initial and final values. The initial mass represents the mass of an object at a starting point, while the final mass captures its mass at a later point. This distinction is essential for calculating accurate percent change in mass.
Units of mass, such as grams (g) and kilograms (kg), play a vital role in these calculations. To ensure accuracy, it may be necessary to convert between units. For example, if the initial mass is measured in grams and the final mass in kilograms, convert the initial mass to kilograms before calculating the percent change. This ensures that both the initial and final masses are expressed in uniform units.
Converting Between Mass Units
Below is a table to facilitate conversions between common mass units:
| Unit | Conversion |
|---|---|
| 1 kilogram (kg) | = 1000 grams (g) |
| 1 gram (g) | = 0.001 kilograms (kg) |
For instance, to convert 500 grams to kilograms, divide 500 by 1000, resulting in 0.5 kilograms. Conversely, to convert 2.5 kilograms to grams, multiply 2.5 by 1000, yielding 2,500 grams.
By understanding the importance of distinguishing between initial and final mass and the ability to convert between mass units, you can accurately calculate percent change in mass, a crucial concept in science and engineering.
Percent Change: Key Concepts
- Explain percentage as a part of a whole expressed as a fraction of 100
- Define fractions and ratios
- Introduce the concept of percent change in mass
Percentage Change: A Deeper Dive into Key Concepts
To truly grasp the concept of percentage change in mass, we need to delve into its fundamental building blocks. Let’s start by exploring what percentage means.
Percentages: A Fraction of 100
A percentage is a way of expressing a part of a whole as a fraction of 100. For instance, 50% means that a part is half of the whole. To understand this further, let’s look at fractions and ratios.
Fractions and Ratios: Understanding Parts and Relationships
A fraction represents a part of a whole as a numerator over a denominator. For example, 1/2 represents half of a whole. A ratio, on the other hand, compares two values by dividing one by the other. For instance, a ratio of 1:2 means that one value is half the size of the other.
Percent Change in Mass: A Measure of Change
Now, let’s apply these concepts to the context of mass. Percent change in mass is a measure that quantifies how much the mass of an object has changed relative to its original mass. This change can be an increase or a decrease, represented by a positive or negative percentage, respectively.
Formula for Calculating Percent Change in Mass
When studying changes in the mass of an object, it’s crucial to understand the concept of percent change in mass. This formula provides a precise method for quantifying the extent to which an object’s mass has increased or decreased.
The formula for percent change in mass is:
Percent Change = [(Final Mass – Initial Mass) / Initial Mass] x 100
Let’s break down this formula into its components:
- Final Mass: This is the mass of the object at the end of the experiment or process.
- Initial Mass: This is the mass of the object at the beginning of the experiment or process.
To calculate the percent change in mass, you simply follow these steps:
- Subtract the initial mass from the final mass: This gives you the change in mass.
- Divide the change in mass by the initial mass: This expresses the change in mass as a fraction of the initial mass.
- Multiply the result by 100: This converts the fraction to a percentage.
For example, if an object initially has a mass of 100 grams and its final mass is 110 grams, the percent change in mass would be:
Percent Change = [(110 grams - 100 grams) / 100 grams] x 100
= (10 grams / 100 grams) x 100
= **10%**
This means that the object’s mass increased by 10% during the experiment or process.
Calculating Percent Change in Mass
- Guide readers through the steps involved in calculating percent change:
- Determining initial and final mass
- Substituting values into the formula
- Multiplying the result by 100
Calculating Percent Change in Mass: A Step-by-Step Guide
Understanding percent change in mass is crucial in various scientific fields, from chemistry to physics. To calculate it accurately, follow these simple steps:
1. Determine Initial and Final Mass
Gather the initial mass (mass at the beginning) and final mass (mass at the end) of the substance. Ensure that the masses are measured in the same units, such as grams or kilograms.
2. Substitute Values into the Formula
Use the formula: Percent Change = [(Final Mass – Initial Mass) / Initial Mass] x 100
3. Multiply the Result by 100
Multiply the calculated value by 100 to convert it from a decimal to a percentage.
Example:
Suppose you want to determine the percent change in mass when a 100-gram sample loses 20 grams of water through evaporation.
1. Determine Initial and Final Mass
- Initial mass = 100 grams
- Final mass = 100 grams – 20 grams = 80 grams
2. Substitute Values into the Formula
- Percent Change = [(80 – 100) / 100] x 100 = -20%
3. Multiply the Result by 100
- Percent Change = -20 x 100 = -20%
Therefore, the percent change in mass after evaporation is -20%, indicating a decrease in mass.
Examples of Percent Change in Mass: Witnessing the Transformations of the World
In the realm of science, the concept of percent change in mass plays a pivotal role in understanding the myriad transformations that occur in our surroundings. From the subtle evaporation of water to the explosive combustion of fuels, this calculation unveils the fascinating changes that substances undergo.
-
Evaporation of Water: As morning breaks, droplets of water glistening on blades of grass begin their journey skyward. The sun’s warm embrace causes molecules to gain energy and break free from the liquid’s surface. In this process of evaporation, the mass of the water droplet steadily decreases as molecules drift away into the atmosphere. The percent change in mass reflects the extent of this transformation.
-
Combustion Reactions: When wood crackles in a bonfire, a mesmerizing chemical dance takes place. Oxygen reacts with the fuel, releasing energy and transforming the wood into carbon dioxide and water vapor. As the wood burns, its mass noticeably diminishes. The percent change in mass quantifies the extent of this combustion, providing insights into the efficiency of the reaction.
-
Chemical Reactions: In a chemistry laboratory, a delicate ballet of atoms unfolds as chemicals interact. Consider the reaction between sodium and chlorine to form sodium chloride. Initially, the mass of the separate elements is known. As they combine, a new compound is created, and the total mass undergoes a shift. The percent change in mass elucidates the intricacies of this chemical transformation.
These examples showcase the diverse applications of percent change in mass, from unraveling the dynamics of everyday phenomena to unlocking the mysteries of complex chemical reactions. Embracing this concept empowers us to delve deeper into the fascinating transformations that shape our world.
Calculating Percent Change in Mass: A Comprehensive Guide
In various scientific disciplines, understanding the concept of percent change in mass is crucial. From chemistry to physics, it plays a vital role in analyzing changes in mass during reactions or processes. This article aims to provide a comprehensive guide to help you grasp this concept, including its formula, calculation steps, and practical examples.
Understanding Mass and Weight
Before delving into percent change, it’s essential to differentiate between mass and weight. Mass is the measure of the amount of matter in an object, while weight is the force exerted on an object due to gravity. The SI unit for mass is kilograms (kg), while the unit for weight is newtons (N).
Initial and Final Mass
To determine the percent change in mass, you need to know the initial mass and the final mass. The initial mass is the mass of the object before the change occurs, and the final mass is the mass after the change. It’s important to ensure that both masses are expressed in the same unit, typically grams or kilograms.
Percent Change: Key Concepts
Percentage is a fraction of 100 that expresses a part of a whole. Fractions and ratios are fundamental concepts in understanding percentages. Percent change in mass refers to the change in mass expressed as a percentage of the initial mass.
Formula for Percent Change in Mass
The formula for calculating percent change in mass is given by:
Percent Change = [(Final Mass - Initial Mass) / Initial Mass] x 100
Calculating Percent Change in Mass
To calculate the percent change in mass, follow these steps:
- Determine the initial mass and the final mass.
- Substitute these values into the formula.
- Calculate the difference between the final mass and the initial mass.
- Divide the difference by the initial mass.
- Multiply the result by 100 to express the answer as a percentage.
Examples of Percent Change in Mass
Percent change in mass finds applications in various fields, including:
- Evaporation of water: Water loses mass as it changes from liquid to gas, resulting in a percent change in mass.
- Combustion reactions: Burning fuels releases energy and produces lighter products, causing a decrease in mass and resulting in a negative percent change in mass.
- Chemical reactions: Chemical reactions involve changes in mass as reactants and products have different compositions, leading to a percent change in mass.
Additional Tips
- Use consistent units: Ensure that both initial and final masses are expressed in the same unit to avoid errors.
- Round answers appropriately: Round your answers to a reasonable number of decimal places, depending on the accuracy of your measurements.
- Use calculators or spreadsheets: Calculators and spreadsheets can help simplify the calculations and improve accuracy and efficiency.