Unveiling The Orbital Overlap Of Cumulene: Insights Into Conjugation
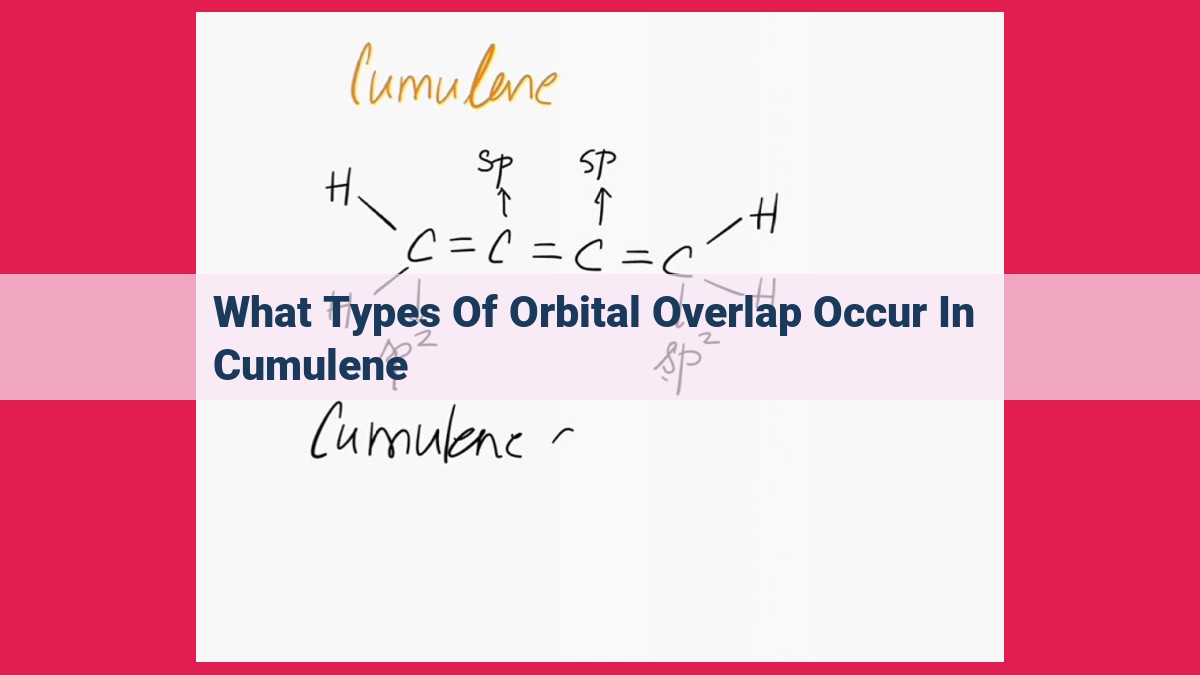
Orbital overlap in cumulene involves the interaction of atomic orbitals to form covalent bonds. σ overlap occurs between sp-sp and sp2-sp orbitals, resulting in single σ-bonds. π overlap occurs between sp2-sp2 orbitals, forming double π-bonds. sp2-sp overlap contributes to the stability of cumulene. Understanding orbital overlap in cumulene provides insights into the bonding, reactivity, and properties of conjugated systems.
- Explain the concept of orbital overlap and its importance in bonding.
- Introduce cumulene as an example of a molecule with multiple types of orbital overlap.
Orbital Overlap: The Foundation of Chemical Bonds
In the realm of chemistry, understanding the concept of orbital overlap is essential. It describes how atomic orbitals, the electron clouds surrounding atoms, interact to form chemical bonds. The strength and type of bond depend on the extent and nature of this overlap.
Cumulene, a fascinating molecule, serves as an excellent example to illustrate the significance of orbital overlap. This molecule features multiple carbon-carbon double bonds, providing a unique platform to study different types of orbital overlap and their consequences.
Types of Orbital Overlap: The Dance of Electron Clouds
Orbital overlap occurs when two or more atomic orbitals overlap each other, allowing electrons to be shared between atoms. The nature of this overlap determines the type of bond formed.
-
Sigma (σ) Overlap: Occurs when the orbitals overlap along the internuclear axis, forming a cylindrical-shaped electron cloud. This type of overlap results in strong and symmetrical single bonds.
-
Pi (π) Overlap: Occurs when the orbitals overlap sideways, perpendicular to the internuclear axis, forming a dumbbell-shaped electron cloud. This type of overlap leads to double and triple bonds.
In cumulene, we encounter both σ and π overlap. The carbon-carbon bonds involve sp-sp overlap (forming single σ bonds) and sp2-sp2 overlap (forming double π bonds). The carbon-hydrogen bonds involve sp2-sp overlap, contributing to the stability of the molecule.
Consequences of Orbital Overlap in Cumulene: Shaping Molecular Properties
The specific types of orbital overlap in cumulene have profound consequences on its molecular properties. The sp-sp overlap in the carbon-carbon bonds provides rigidity to the molecule’s backbone. The sp2-sp2 overlap in the carbon-carbon double bonds allows for rotational freedom, contributing to the molecule’s flexibility.
Additionally, the sp2-sp overlap in the carbon-hydrogen bonds provides stability to the molecule by delocalizing electrons and reducing the energy of the system.
Applications of Orbital Overlap in Cumulene: Unlocking New Horizons
Understanding orbital overlap in cumulene has opened up exciting avenues for research and applications. Cumulene compounds are found in various natural products and have potential applications in materials science and pharmaceuticals. By manipulating the orbital overlap, scientists can design new materials with tailored properties and develop novel drugs with improved efficacy.
In conclusion, orbital overlap is a fundamental concept in chemistry that plays a crucial role in shaping molecular properties and determining chemical bonding. Cumulene, with its diverse types of orbital overlap, provides a compelling example to illustrate this concept and its far-reaching significance in the scientific world.
Types of Orbital Overlap
In the realm of molecular bonding, the concept of orbital overlap plays a pivotal role. Orbital overlap occurs when the electron clouds of two atoms overlap, leading to the formation of a chemical bond. The extent and type of overlap determine the strength and nature of the bond.
Sigma (σ) overlap occurs when the electron clouds of two atoms overlap head-to-head. This type of overlap is found in single bonds, such as the C-C bond in ethane (H3C-CH3) or the H-H bond in hydrogen (H2). In σ overlap, the electron density is concentrated along the internuclear axis, resulting in a strong, stable bond.
Pi (π) overlap occurs when the electron clouds of two atoms overlap side-by-side. This type of overlap is found in double bonds, such as the C=C bond in ethene (H2C=CH2) or the p orbitals in ozone (O3). In π overlap, the electron density is concentrated above and below the internuclear axis, forming a less stable bond compared to σ overlap.
Sigma-pi (σ-π) overlap is a combination of σ and π overlap. It occurs when one electron cloud overlaps head-to-head and the other overlaps side-by-side. This type of overlap is found in conjugated systems, where alternating single and double bonds allow for the delocalization of electrons. σ-π overlap enhances the stability of conjugated systems by lowering their overall energy.
Finally, pi-pi (π-π) overlap occurs when the electron clouds of two π orbitals overlap side-by-side. This type of overlap is found in extended π-systems, such as those found in aromatic compounds like benzene (C6H6). π-π overlap leads to the formation of resonance structures and contributes to the unique properties of aromatic compounds.
Specific Types of Orbital Overlap in Cumulene
In our journey into the intricacies of orbital overlap in cumulene, we’ll now delve into the specific types of overlap that define its unique bonding characteristics.
sp-sp Overlap in C-C Bonds
The carbon-carbon bonds in cumulene, represented by C-C, are formed through the overlap of sp hybrid orbitals. These orbitals result from the mixing of one s-orbital and one p-orbital. The head-to-head overlap of the sp orbitals creates a single σ-bond. This σ-bond provides the backbone of the cumulene molecule.
sp2-sp2 Overlap in C=C Bonds
The carbon-carbon double bonds in cumulene, denoted as C=C, are formed via the overlap of sp2 hybrid orbitals. Sp2 hybridization occurs when two p-orbitals and one s-orbital combine. The side-by-side overlap of the sp2 orbitals creates a π-bond. This π-bond reinforces the C=C double bond and allows for free rotation around the bond axis.
sp2-sp Overlap in C=C and C-H Bonds
The remaining π-bonds in cumulene are formed through the overlap of sp2 orbitals from the carbon atoms with sp orbitals from the hydrogen atoms. This overlap is weaker than the sp2-sp2 overlap due to the difference in orbital energies. It provides additional stability to the cumulene molecule and influences its electronic properties.
**Consequences of Orbital Overlap in Cumulene: Unraveling the Secrets of Bonding**
Cumulene, a fascinating molecule with alternating single and double bonds, showcases the intricate interplay of orbital overlaps. These overlaps, like puzzle pieces locking together, determine the unique characteristics and stability of this remarkable compound.
Formation of Single σ-Bonds
The single bonds in cumulene arise from sp-sp overlap. Imagine two sp hybridized carbon atoms, each with one s and one p orbital. As these atoms approach each other, their s orbitals overlap head-to-head, forming a strong, symmetrical σ-bond that holds the carbon atoms together. This sigma bond is the cornerstone of the cumulene backbone, providing the structural integrity necessary for its existence.
Formation of Double π-Bonds
In contrast to the single bonds, the double bonds in cumulene result from sp2-sp2 overlap. Here, sp2 hybridized carbon atoms, each possessing three sp2 orbitals and one p orbital, come into play. The sp2 orbitals overlap side-by-side, forming two equivalent π-bonds, perpendicular to each other. These π-bonds reinforce the structural integrity of the molecule, creating a rigid, planar geometry that distinguishes cumulene from other carbon-based molecules.
Role of sp2-sp Overlap in Stability
Beyond the formation of bonds, sp2-sp overlap also plays a crucial role in the stability of cumulene. The sp2 orbitals involved in this overlap have higher energy than sp orbitals but lower energy than p orbitals. This unique electronic configuration results in resonance, a phenomenon where electrons can delocalize across multiple atomic centers. The delocalization of electrons in cumulene stabilizes the molecule by distributing the electron density more evenly, making it less reactive and more resistant to breaking apart.
Examples and Applications of Orbital Overlap in Cumulene
The concept of orbital overlap and its consequences in cumulene molecules opens up a fascinating world of applications in various fields.
Real-Life Examples of Cumulene Compounds
Cumulene compounds are found in a variety of natural and synthetic materials. One notable example is carbodiimide, a highly reactive compound used in the synthesis of pharmaceuticals and agrochemicals. Another is carbodiphosphorane, used as a reagent in organic chemistry reactions.
Properties of Cumulene Compounds
The unique orbital overlap patterns in cumulenes give rise to distinct properties. Cumulenes often exhibit high reactivity, making them valuable intermediates in organic synthesis. They also possess interesting optical properties, leading to their potential use in optoelectronics and sensor technologies.
Design of New Materials and Pharmaceuticals
Understanding orbital overlap in cumulenes is crucial for the rational design of new materials and pharmaceuticals. By manipulating the types and extent of orbital overlap, scientists can tailor the electronic properties of materials and create compounds with specific functionalities.
For instance, in the pharmaceutical industry, orbital overlap can be engineered to optimize the binding interactions of drug molecules with target proteins. This knowledge can accelerate the discovery and development of more effective and selective drugs.
The study of orbital overlap in cumulenes provides a powerful tool for understanding the behavior of molecules and designing novel materials and pharmaceuticals. As research continues, the full potential of orbital overlap in cumulene chemistry awaits further exploration, offering promising avenues for innovation and scientific breakthroughs.