Unlocking Orbital Electron Capacity: Key To Understanding Atomic Structure
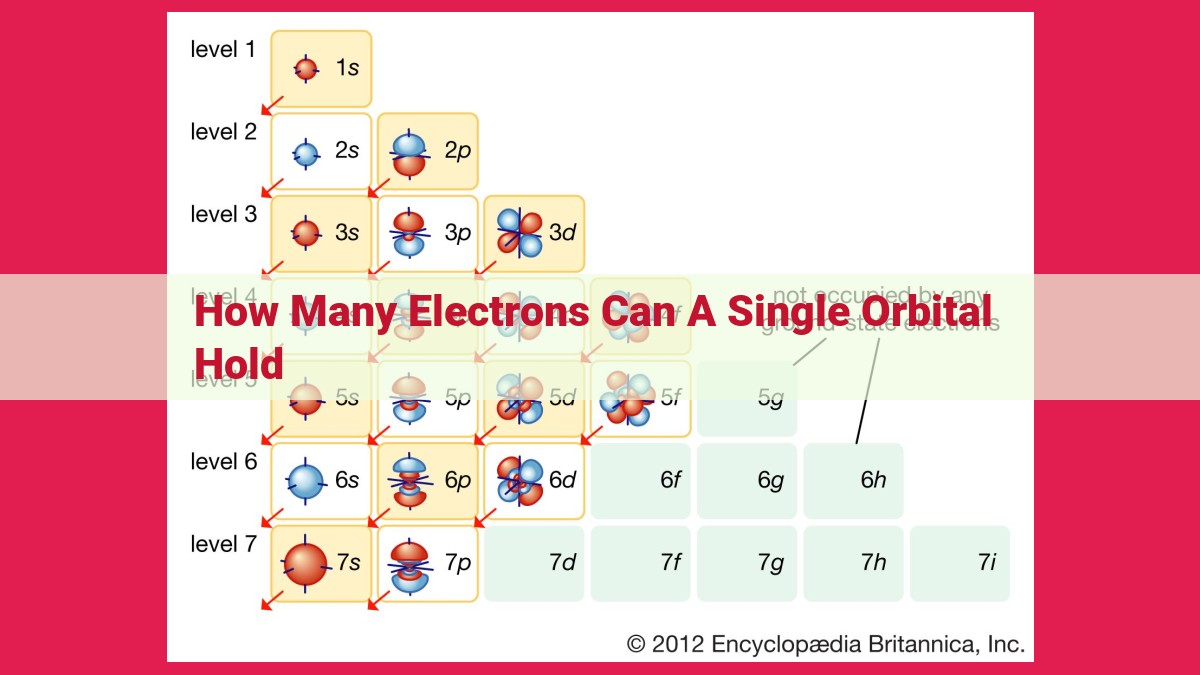
The electron capacity of an orbital, dictated by the Aufbau and Pauli Exclusion Principles, determines how many electrons it can hold. In each subshell (s, p, d, or f), defined by its shape and energy level, orbitals exist in groups. The electron capacity of a subshell is given by the formula 2n², where n is the energy level. For instance, in the first energy level, the 1s subshell can hold a maximum of 2 electrons (n=1), while the 2p subshell can accommodate up to 6 electrons (n=2). Understanding orbital electron capacity is crucial in comprehending atomic structure and chemical bonding.
Electron Orbitals: Unraveling the Quantum Dance of Electrons
Imagine electrons dancing around the nucleus of an atom, each with its own unique address, like tiny houses within an atomic neighborhood. But what determines the size of these electronic dwellings? The answer lies in understanding the electron capacity of orbitals, the regions where electrons are most likely to be found.
In this blog post, we’ll journey into the quantum realm to explore the fundamental principles that govern electron occupancy and discover the significance of this concept in unraveling the secrets of atomic structure and chemical bonding.
Fundamental Principles: A Quantum Symphony
Like a musical symphony, the behavior of electrons follows a set of quantum rules. The Aufbau Principle dictates that electrons gracefully fill orbitals based on increasing energy levels, like notes ascending a musical scale. The Pauli Exclusion Principle, a cosmic dance choreographer, ensures that no two electrons share the same quantum state, preventing electronic pile-ups.
Structure of Atoms: A Matryoshka of Energy
Atoms resemble intricate Matryoshka dolls, with electrons occupying concentric shells or energy levels. Each shell harbors subshells or groups of orbitals with different shapes, like tiny rooms within a house. Orbitals, the atomic equivalent of electron apartments, are defined by the dance of electrons within them.
Electron Capacity of Orbitals: Determining Occupancy
The electron capacity of an orbital, like the maximum number of guests an apartment can hold, is a fundamental property. The formula (2n²) determines the occupancy limit, where n represents the principal quantum number of the shell. Different types of orbitals—s, p, d, f—have varying capacities.
Understanding orbital electron capacity is crucial for deciphering atomic structures and unraveling the mysteries of chemical bonding. It enables us to predict electron arrangements, influencing the way atoms interact and form molecules, the building blocks of our world.
In essence, electrons are not mere tenants within atoms; they are integral players in the atomic symphony, orchestrating the very essence of matter itself. Their dance within orbitals, governed by quantum principles, shapes the blueprint of our physical world, making the study of orbital electron capacity an enlightening pursuit.
Understanding the Electron Capacity of Orbitals: A Fundamental Principle
Electrons, the tiny particles that orbit the nucleus of an atom, play a pivotal role in shaping the chemical behavior of elements. One crucial aspect of understanding electrons is comprehending the electron capacity of orbitals – the maximum number of electrons that can reside within a specific orbital. This concept lays the foundation for unraveling the intricate structure of atoms and their ability to form chemical bonds.
The Aufbau Principle: A Hierarchical Filling Pattern
Imagine orbitals as designated parking spots for electrons within an atom. The Aufbau Principle dictates the order in which these parking spots are filled, akin to a hierarchical queuing system. Electrons occupy orbitals starting from the lowest energy levels, gradually filling higher energy levels as atomic number increases. This logical progression ensures that electrons occupy the most stable and energetically favorable positions.
The Pauli Exclusion Principle: A Matter of Identity
Electrons, like individuals, possess unique identities. The Pauli Exclusion Principle proclaims that no two electrons within an atom can have the exact same set of quantum numbers – a combination of properties that defines their energy state, shape, and orientation. This exclusivity principle maintains order in the electron distribution, preventing overcrowding and ensuring that each electron has its own distinct place within the atomic realm.
Structure of Atoms:
- Shells: Describe shells as groups of orbitals with similar energy levels.
- Subshells: Explain subshells as groups of orbitals with different shapes within a shell.
- Orbitals: Define orbitals as regions where electrons are likely to be found.
Understanding the Structure of Atoms: Shells, Subshells, and Orbitals
In the heart of every atom lies a complex arrangement of electrons orbiting the nucleus. To grasp the behavior of these electrons, we delve into the intricacies of atomic structure, a fundamental concept in chemistry.
Shells: Energy Levels in the Atom
Imagine the atom as a miniature solar system, where electrons orbit the nucleus like planets around a star. These electrons occupy specific shells, which are energy levels designated by numbers: 1, 2, 3, and so on, starting from the nucleus. Each shell represents a range of energy, with lower shells being closer to the nucleus and having lower energy.
Subshells: Orbitals within Shells
Within each shell lie subshells, smaller regions of specific shapes where electrons reside. These subshells are denoted by letters: s, p, d, and f. Each subshell has a distinct energy and shape. For instance, s subshells are spherical, while p subshells resemble dumbbells.
Orbitals: Where Electrons Dance
Finally, within each subshell are orbitals, well-defined regions where electrons are most likely to be found. Orbitals are like three-dimensional clouds that surround the nucleus. Each orbital can hold a maximum of two electrons, a principle known as the Pauli Exclusion Principle.
Understanding the arrangement of electrons in shells, subshells, and orbitals is crucial for comprehending atomic behavior. These concepts form the foundation for understanding chemical bonding, the forces that hold atoms together to form molecules, and the properties of various elements that shape our world.
Electron Capacity of Orbitals: Unraveling the Secret Code of Atoms
** delve into the enigmatic world of atoms, where electrons dance around the nucleus in a celestial ballet. Each electron occupies a specific orbital, a region where it’s most likely to be found. But how many electrons can an orbital accommodate? Unraveling this mystery is crucial for understanding the building blocks of matter.**
Formula for Electron Capacity: 2n²
The electron capacity of an orbital is dictated by a simple formula: 2n². Here, n represents the principal quantum number of the orbital, which indicates its energy level.
- 1s orbital (n=1): Can accommodate a maximum of 2 electrons.
- 2s orbital (n=2): Can accommodate a maximum of 2 electrons.
- 2p orbitals (n=2): Can accommodate a maximum of 6 electrons (3*2).
- 3s orbital (n=3): Can accommodate a maximum of 2 electrons.
- 3p orbitals (n=3): Can accommodate a maximum of 6 electrons.
- 3d orbitals (n=3): Can accommodate a maximum of 10 electrons.
- 4s orbital (n=4): Can accommodate a maximum of 2 electrons.
- 4p orbitals (n=4): Can accommodate a maximum of 6 electrons.
Types of Orbitals and Their Electron Capacities
Orbitals are classified into different types based on their shape and orientation.
- s orbitals: Spherical in shape, they have only one subshell and can accommodate a maximum of 2 electrons.
- p orbitals: Dumbbell-shaped, they have three subshells and can accommodate a maximum of 6 electrons.
- d orbitals: Complex shapes, they have five subshells and can accommodate a maximum of 10 electrons.
- f orbitals: More complex shapes than d orbitals, they have seven subshells and can accommodate a maximum of 14 electrons.
Understanding the electron capacity of orbitals is fundamental to comprehending atomic structure and chemical bonding. The formula 2n² empowers us to predict the maximum number of electrons that can reside in each orbital, providing a deeper insight into the intricate dance of electrons around the atomic nucleus. This knowledge serves as a cornerstone for unraveling the mysteries of the chemical world and unlocking the secrets of matter.
Demystifying the Capacity of Orbitals: A Journey into the Realm of Electrons
Have you ever wondered how many electrons can cozy up in an atomic orbital? Prepare to embark on a captivating exploration of the electron capacity of orbitals.
Fundamental Principles
Understanding orbital electron capacity hinges on two fundamental principles:
- Aufbau Principle: Electrons occupy orbitals with the lowest energy levels first.
- Pauli Exclusion Principle: No two electrons can share the same quantum state (a unique combination of energy, spin, and shape).
Structure of Atoms
Atoms are composed of a nucleus surrounded by a cloud of electron-occupied regions called orbitals. These orbitals are arranged in concentric shells, each containing subshells (regions with distinct shapes).
Electron Capacity of Orbitals
The formula for determining the maximum number of electrons in a subshell is 2n², where n represents the number of the energy level (or shell).
Different types of orbitals have varying electron capacities:
- s-orbitals: Hold a maximum of 2 electrons.
- p-orbitals: Hold a maximum of 6 electrons (three pairs).
- d-orbitals: Hold a maximum of 10 electrons (five pairs).
- f-orbitals: Hold a maximum of 14 electrons (seven pairs).
Example Application
Consider the 1s subshell in the first energy level (n = 1). Using the formula 2n², we can determine its electron capacity:
2 × (1)² = 2
Therefore, the 1s subshell can accommodate up to 2 electrons.
Understanding orbital electron capacity is crucial for deciphering atomic structure, predicting chemical bonding behavior, and comprehending the behavior of matter. So, there you have it—a voyage into the enigmatic world of orbital electron capacity.