Mastering The Octet Rule And Duplet Rule: Achieving Electron Shell Stability

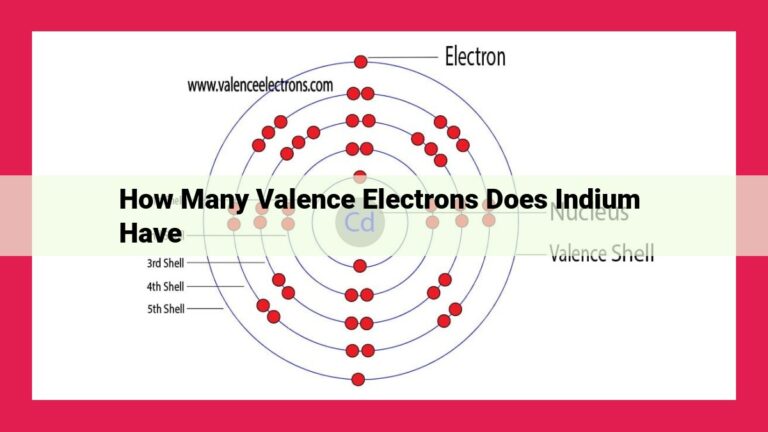
Most stable atoms have a full valence electron shell, which means they have the same number of valence electrons as a noble gas. The octet rule states that atoms are most stable when they have eight valence electrons, while the duplet rule applies to helium and hydrogen atoms, which are stable with two valence electrons. Noble gas electron configurations are considered stable because they have a full valence shell, and atoms tend to gain, lose, or share electrons to achieve this stable configuration.
The Hidden Power of Valence Electrons: Unlocking the Secrets of Stable Atoms
In the realm of chemistry, stability is a precious commodity. Atoms, the building blocks of matter, strive to achieve this elusive state, and the key to their success lies in an enigmatic group of electrons known as valence electrons.
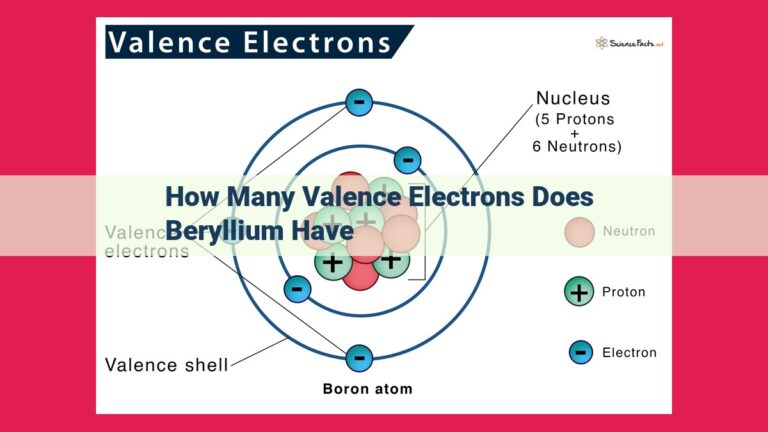
Valence electrons are the outermost electrons in an atom’s electron configuration, and they play a pivotal role in determining its stability. Just like a house with a solid foundation, atoms with a stable valence electron configuration possess a strong foundation that allows them to exist in harmony.
Imagine an atom as a miniature solar system, with the nucleus acting as the sun and the electrons orbiting around it like planets. Valence electrons, positioned in the outermost orbit, are particularly influential in determining the atom’s stability because they are the most likely to interact with other atoms.
When atoms interact, their valence electrons come into play, shaping the chemical bonds that hold them together. These electrons can be shared, transferred, or lost, giving rise to the vast array of molecules and compounds that make up our world.
Understanding valence electrons is crucial for unraveling the tapestry of chemical behavior. They are the gateway to understanding why certain elements are highly reactive while others remain aloof, why some substances form strong bonds while others break apart effortlessly.
Delving into the world of valence electrons is a fascinating journey that unveils the secrets of atomic stability and chemical reactivity. By embracing their power, we can unlock a deeper appreciation of the intricate workings of our universe.
The Octet Rule: Achieving Electron Stability
In the realm of chemistry, valence electrons play a pivotal role in determining the stability of atoms. These electrons occupy the outermost energy level and wield the power to influence an atom’s chemical behavior. One of the most fundamental principles governing valence electrons is known as the octet rule.
The octet rule states that atoms tend to achieve stability by acquiring or losing electrons to attain a full set of eight valence electrons. This stable configuration resembles the electron configuration of noble gases, the most chemically inert elements in the periodic table.
Atoms with less than eight valence electrons often readily participate in chemical bonding to gain or share electrons until they reach the coveted octet. This electron-hungry behavior drives atoms to interact and form molecules and compounds.
For instance, sodium (Na) has a single valence electron. To achieve stability, it readily gives up this electron to achieve an electron configuration resembling the stable neon atom (Ne). On the other hand, chlorine (Cl) has seven valence electrons. It requires just one more electron to complete its octet, which it often obtains by sharing electrons with other atoms.
The octet rule helps explain why some elements form bonds in specific ways. For example, sodium and chlorine form ionic bonds by transferring electrons. Sodium loses an electron and becomes a positively charged cation, while chlorine gains an electron and becomes a negatively charged anion. This transfer creates a strong electrostatic attraction between the ions, forming a stable compound.
The octet rule is not an absolute law, but it provides a valuable framework for understanding the chemical behavior of elements. It serves as a testament to the fundamental principle that atoms strive to achieve stability and that the octet configuration is the ultimate goal for electron configurations.
The Duplet Rule: A Path to Atomic Stability
In the realm of chemistry, valence electrons hold a profound significance in shaping the stability and behavior of atoms. These are the electrons that reside in the outermost energy level of an atom and play a pivotal role in determining its chemical reactivity. Among the fundamental principles governing valence electrons is the Duplet Rule.
The Duplet Rule applies to atoms with small atomic numbers, particularly those in Group 18 of the periodic table. These atoms, like helium (He), possess a stable electron configuration with only two electrons in their outermost energy level, known as the duplet configuration.
The reason for this stability lies in the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of quantum numbers. This means that the two electrons in a duplet configuration must have opposite spins, canceling out each other’s magnetic fields and minimizing the energy of the atom.
Interestingly, the Duplet Rule is closely connected to the Octet Rule, which governs the stability of larger atoms. The Octet Rule states that atoms tend to achieve a stable configuration by having eight valence electrons. However, in the case of small atoms with helium-like electron configurations, a duplet configuration is sufficient for stability.
This connection between the Duplet Rule and the Octet Rule can be understood by considering the electronic structure of noble gases. Noble gases, such as helium (He) and neon (Ne), have stable electron configurations with either two (duplet) or eight (octet) valence electrons. This stability arises from the fact that both duplet and octet configurations minimize the potential energy of the atom.
In summary, the Duplet Rule is a fundamental principle that governs the stability of atoms with helium-like electron configurations. It states that these atoms achieve stability by having two electrons in their outermost energy level, forming a duplet configuration. This rule is closely related to the Octet Rule and plays a crucial role in determining the chemical behavior of these atoms. By understanding the Duplet Rule, we gain valuable insights into the underlying principles that shape the world of chemistry.
Noble Gas Electron Configurations: The Blueprint for Stability
In the realm of atoms, stability is paramount, and valence electrons play a pivotal role in this delicate dance. Atoms strive to achieve a harmonious electron configuration, mirroring that of noble gases—the pinnacle of stability in the atomic world.
Noble gases possess a complete outer shell of valence electrons, forming a stable octet. This octet configuration endows these elements with an aversion to chemical reactions, making them inert and highly unreactive.
Striving for this coveted stability, atoms exhibit a strong tendency to gain, lose, or share electrons in order to attain a noble gas electron configuration. By mirroring the octet rule, which dictates that atoms seek to acquire a stable octet of valence electrons, they enhance their stability and minimize their reactivity.
This noble gas electron configuration serves as a roadmap for stability. It guides atoms in their pursuit of harmonious electron arrangements, shaping their chemical properties and influencing their reactivity in the intricate dance of the chemical world.
Valence Electrons: The Key to Atomic Stability
Every atom’s behavior and properties are dictated by its valence electrons, the electrons residing in the outermost energy level. These electrons play a crucial role in determining atomic stability, influencing how elements interact with each other. Understanding the significance of valence electrons unravels the secrets of chemical bonding and reactivity.
Stability and Valence Electrons
Definition of Stability
In the world of atoms, stability refers to electron configurations that minimize the atom’s energy. Atoms crave a state of low energy, and their valence electrons hold the key to achieving this stability.
Influence of Valence Electrons on Stability
The number and arrangement of valence electrons significantly impact atomic stability. Atoms with a full or empty outermost energy level are the most stable because they possess the lowest energy configurations.
Octet and Duplet Rule
The octet rule dictates that atoms tend to gain or lose electrons to attain a stable octet of valence electrons (eight electrons in the outermost energy level). This configuration mirrors the electron configuration of noble gases, the most stable elements. Atoms with a complete octet have low reactivity and form bonds only under specific conditions.
In contrast, the duplet rule applies to atoms with only one or two valence electrons. These atoms achieve stability by forming covalent bonds with other atoms, sharing electrons to create a stable pair. Helium, with its filled duplet, exemplifies this rule.
Valence electrons are the driving force behind atomic stability. Their number and arrangement dictate the stability of atoms, influencing their reactivity and the formation of chemical bonds. Understanding the significance of valence electrons unlocks a deeper comprehension of chemical behavior and the fascinating world of atomic interactions.
Valence Electrons: The Gatekeepers of Chemical Behavior
Picture an atom as a tiny world, with a bustling nucleus at the center, surrounded by a cloud of electrons. These electrons aren’t all created equal; the valence electrons in the outermost shell are the ones that really make the action happen.
What Do Valence Electrons Do?
Valence electrons are the key players in chemical bonding—the process that holds atoms together to form molecules. They’re like little social butterflies, fluttering around, looking for other atoms to dance with.
The number of valence electrons an atom has determines its chemical properties—how it reacts with other elements. Elements with similar valence electron configurations tend to have similar chemical behavior, forming the basis of the periodic table.
The Magic Number: 8
In the atomic world, there’s a special number that seems to bring stability: 8. Atoms that have 8 valence electrons, like the noble gases, are the ultimate loners—they’re happy and stable all on their own, rarely forming bonds with other atoms.
Valence Electron Rules
To achieve this stability, atoms follow certain rules:
- The Octet Rule: Most atoms want to have 8 valence electrons, either by sharing them with other atoms or gaining/losing them outright.
- The Duplet Rule: Atoms with only two valence electrons (like helium) are stable with that configuration.
Finding Your Valence Electrons
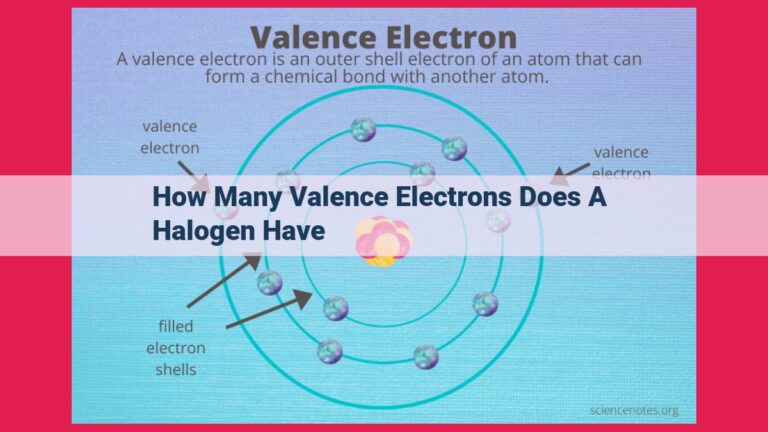
To find the valence electrons of an element, look at its position in the periodic table:
- Groups 1-2: 1-2 valence electrons
- Groups 13-17: 3-7 valence electrons
- Group 18 (noble gases): 8 valence electrons
So, there you have it—valence electrons, the unsung heroes of the atomic world, shaping the chemical world we see around us. Understanding them unlocks the secrets of chemical bonding and reactivity, allowing us to unravel the mysteries of matter on the atomic scale.