The Nucleus: The Core Of The Atom And Its Importance In Chemistry And The Universe
The Nucleus: The Heart of the Atom
At the heart of every atom lies the nucleus, a dense region containing protons and neutrons. Protons, with their positive charges, determine the atomic number and influence chemical reactions. Neutrons, lacking electrical charge, provide mass and play crucial roles in nuclear reactions and radioactivity. This central hub governs the physical and chemical properties of elements, shaping the behavior of matter and the very fabric of our universe.
Atoms: The Fundamental Building Blocks of Matter
In the realm of science, the atom stands as the foundational unit of all matter. A mystical entity, it forms the very essence of everything that exists around us, from the chair you sit on to the air you breathe. But what exactly is an atom?
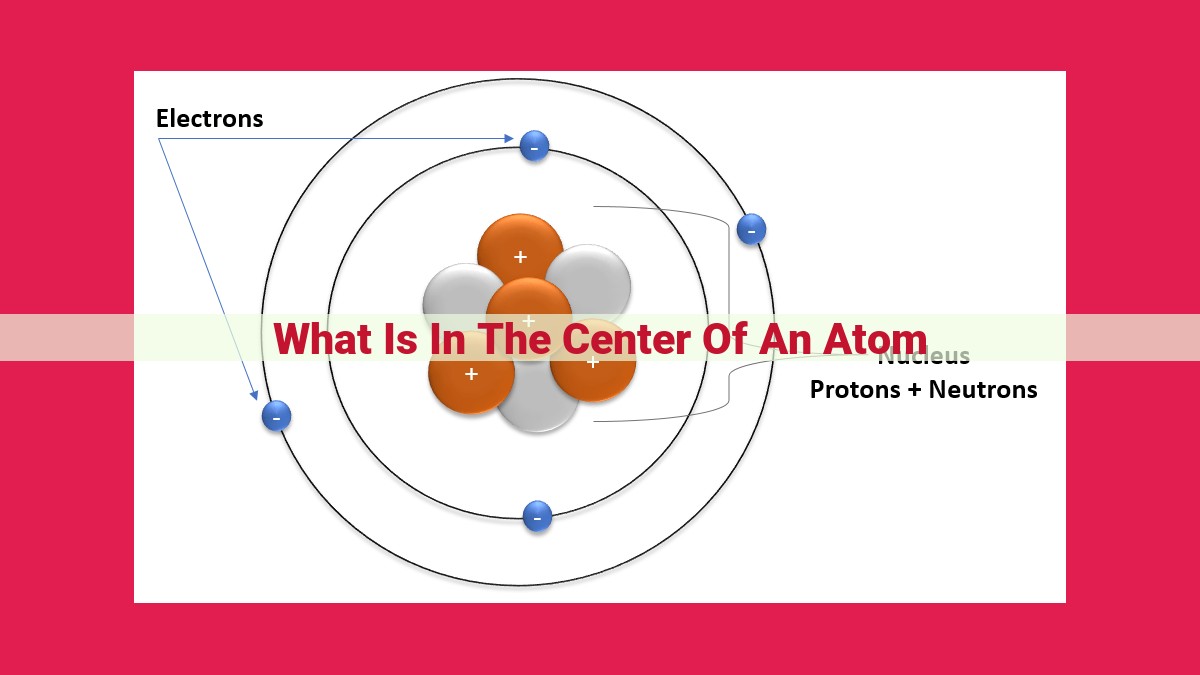
Imagine a tiny, invisible sphere, so small that even a billion of them would fit on the tip of a single strand of hair. This atomic structure is a universe in itself, composed of three fundamental components: electrons, protons, and neutrons.
Electrons, the negatively charged inhabitants of the atom, orbit the nucleus, the positively charged central core like miniature planets circling a sun. Protons, residing within the nucleus, are positively charged and balance the electrons’ negative charge.
Alongside protons, the nucleus also houses neutrons, uncharged particles that contribute to the atom’s atomic mass. The number of protons determines an element’s identity, giving it its unique place on the Periodic Table.
The Nucleus: The Heart of the Atom
In the microscopic realm of atoms, a tiny universe unfolds, with the nucleus serving as its vibrant heart. Deep within the atom’s core, this dense and enigmatic region holds the key to an element’s identity and the power of nuclear reactions.
The nucleus, an infinitesimal speck compared to the atom’s vastness, resides at its very center. A haven of protons and neutrons, it is the atom’s bastion of positive and neutral charges. Protons, with their unfailing positive electric charge, balance the negative charge of electrons orbiting around the nucleus. Neutrons, devoid of charge, act as mediators, stabilizing the nucleus and keeping the protons from repelling each other.
Together, protons and neutrons form a tightly bound unit, held captive by the strong nuclear force. This force, a formidable power that overcomes the repulsive electrostatic force between protons, ensures the nucleus’s integrity. Within this nuclear sanctuary, protons and neutrons mingle freely, giving rise to the diversity of elements in our universe.
Each element possesses a unique nucleus, defined by its number of protons. This number, known as the atomic number, is like a fingerprint, distinguishing one element from another. It determines an element’s chemical properties and its place on the periodic table.
Protons: The Positive Force Driving the Atomic Landscape
In the realm of chemistry, the proton stands as a fundamental pillar, shaping the very essence of every atom. These subatomic particles reside within the nucleus, the atom’s compact and dense core. Protons are positively charged, carrying a fundamental charge that is equal in magnitude but opposite in sign to that of an electron. This inherent positive charge plays a pivotal role in defining the identity, properties, and interactions of all elements.
The atomic number of an element is directly linked to the number of protons it possesses. This unique identifier helps distinguish one element from another, as each element occupies a specific position on the periodic table based on its atomic number. The atomic number also determines the number of electrons that an atom must possess to achieve a neutral charge, ensuring stability.
Protons contribute to the atomic mass of an element. However, their contribution is not as significant as that of neutrons, which have a neutral charge and a slightly larger mass. The combined number of protons and neutrons in an atom’s nucleus determines its mass number, a value that helps identify different isotopes of the same element.
One of the most crucial roles of protons is neutralizing the negative charge of electrons. Electrons, with their inherent negative charge, orbit the nucleus. Without the protons’ positive charge, these electrons would spiral into the nucleus, causing the atom to collapse. Protons, therefore, provide the necessary balance to maintain the atom’s stability and structure.
Furthermore, protons influence the atomic structure and molecular orbital formation. The arrangement of protons and electrons within an atom determines its size, shape, and chemical properties. The interplay between protons and electrons also influences the formation of molecular orbitals, the regions where electrons are most likely to be found in multi-atomic molecules.
In conclusion, protons are indispensable to the very existence and behavior of atoms. Their positive charge shapes atomic identity, mass, and structure. Protons are truly the driving force behind the diverse and fascinating world of chemistry, enabling the formation of elements and molecules that make up everything around us.
Neutrons: The Neutral Partners in the Atomic Nucleus
In the heart of every atom lies the enigmatic nucleus, a dense core packed with protons and neutrons. While protons carry the positive force that defines an element’s identity, neutrons remain the neutral partners, playing a crucial role in nuclear stability and unlocking the secrets of the atomic world.
Neutrons, as their name suggests, possess no electrical charge, making them indifferent to the electrostatic forces that govern proton interactions. This neutrality allows neutrons to reside within the nucleus, balancing the positive charge of protons and ensuring the dynamism and stability of the atom.
The Role of Neutrons in Nuclear Reactions and Radioactivity
Neutrons play a pivotal role in nuclear reactions, particularly in processes like nuclear fission and fusion. During fission, a heavy nucleus splits into two or more smaller nuclei, releasing energy in the form of radiation. The initiation of fission often involves the absorption of a neutron by the unstable nucleus.
In the realm of radioactivity, neutrons also have a profound impact. Some isotopes, or variants of an element with different numbers of neutrons, are radioactive, emitting radiation to achieve stability. The decay of radioactive isotopes can involve the emission of neutrons, further altering the composition of the nucleus.
The Significance of Isotopes and Mass-Energy Equivalence
The presence of neutrons in an atom’s nucleus has a significant impact on its mass. Isotopes of the same element have the same number of protons but different numbers of neutrons. This difference in neutron count results in variations in atomic mass, which can be precisely measured using instruments like mass spectrometers.
Einstein’s famous equation, E=mc², reveals a fundamental relationship between mass and energy. The heavier an isotope, the greater its nuclear mass, and consequently, the greater its potential for energy release through nuclear reactions. This principle forms the basis of nuclear power generation and the development of atomic weapons.
In conclusion, neutrons, the neutral partners within the nucleus, play a crucial role in maintaining atomic stability, influencing nuclear reactions, and unlocking the secrets of radioactivity. Their significance extends beyond the atomic realm, contributing to the understanding of mass-energy equivalence and fueling advancements in nuclear science and technology. As scientists continue to unravel the mysteries of the nucleus, the importance of neutrons will undoubtedly remain paramount in our quest for scientific knowledge and technological progress.