The Nucleus: The Atom’s Core, Its Structure, And Its Influence On Electron Behavior
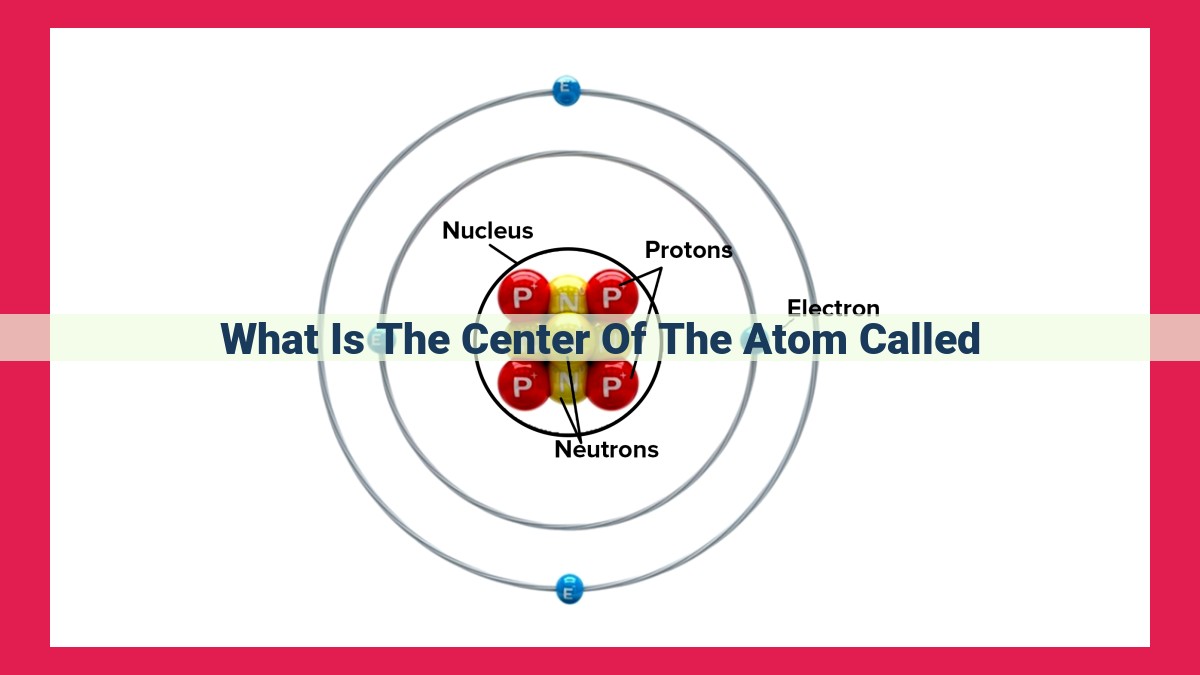
The nucleus, the atom’s heart, resides centrally, housing protons and neutrons. Its mass and positive charge make it the atom’s core, while its location influences electron behavior. The nucleus’s shape, usually spherical, can deform under nuclear forces, affecting stability and reactions.
Delving into the Heart of the Atom
In the vast expanse of our universe, where celestial wonders unfold, there exists a realm of minuscule particles that shape the very fabric of our existence: atoms. These fundamental building blocks hold the blueprint for all that we see, touch, and experience. At the heart of every atom lies a mysterious and captivating region, the nucleus.
This enigmatic core, responsible for an atom’s identity and behavior, beckons us to unravel its secrets. Join us on an extraordinary journey to decode the nucleus, the vital centerpiece that anchors the atomic realm. Let us delve into the depths of these microscopic marvels and uncover the fascinating secrets they hold.
Nucleus: The Mighty Core of the Atom
At the very heart of every atom, nestled amidst a whirlwind of electrons, lies a tiny but mighty core known as the nucleus. This minuscule entity is the very essence of an atom’s identity, determining its mass, electrical charge, and ultimately, its behavior in the world.
The nucleus, a mere speck within the vastness of the atom, packs an enormous punch. It is the repository of an atom’s mass. Protons, with their positive electrical charge, and neutrons, their neutral counterparts, reside within the nucleus, their combined weight accounting for the bulk of an atom’s mass.
The nucleus is not merely a passive repository of mass; it is also the source of an atom’s electrical charge. The protons, with their positive charge, dominate the nucleus, giving the atom its overall positive charge. This electrical charge plays a crucial role in shaping the atom’s interactions with its surroundings, influencing its chemical properties and behavior in electric and magnetic fields.
The Nucleus’s Central Command: Shaping the Atomic Landscape
At the Heart of Matter
The nucleus, the central command of every atom, occupies a strategic position that influences the behavior of the entire atomic realm. Its presence at the core defines the very nature of an atom and underpins its fundamental properties.
Gravity’s Grip
Imagine the nucleus as a miniature sun, anchored at the center of a vast expanse. Its gravitational pull, though minuscule, governs the movement of electrons, the subatomic particles that orbit it like planets. Like celestial bodies circling a star, electrons are drawn towards the nucleus’s positive charge.
Orbital Symphony
The nucleus’s central position dictates the orbital paths of electrons. Electrons occupy specific energy levels, or shells, arranged like concentric rings around the nucleus. The closer an electron is to the nucleus, the tighter its orbit and the stronger the gravitational pull it experiences.
Electron Dance
The nucleus’s gravitational field not only influences electron orbits but also governs their interactions. Electrons constantly repel each other due to their negative charges. However, the nucleus’s positive charge exerts a balancing force, stabilizing the electron cloud and preventing it from collapsing into the nucleus.
The nucleus’s central position is a cornerstone of atomic architecture. Its gravitational pull shapes the orbits of electrons, dictating the atom’s size, shape, and chemical properties. The nucleus is the conductor of the atomic orchestra, orchestrating the harmonious dance of electrons and defining the fundamental nature of matter.
Unveiling the Building Blocks of the Nucleus
At the heart of every atom lies a tiny, dense core known as the nucleus, a realm of immense power and mystery. Within this minuscule space reside the fundamental particles that determine an atom’s identity and properties: protons and neutrons.
Protons and Neutrons: The Core Constituents
Protons, with their positive charge, form the positive core of the nucleus. Their number defines an element’s atomic number, distinguishing it from all others. Neutrons, on the other hand, carry no electrical charge, acting as neutral companions to protons.
The Mass Enigma: Protons vs. Neutrons
While both protons and neutrons contribute to an atom’s mass, their masses differ significantly. Protons are approximately 1,836 times more massive than electrons, while neutrons weigh in at about 1,839 times the electron’s mass. This difference in mass plays a crucial role in determining an atom’s stability and behavior.
The Glue that Binds: Quarks and Binding Energy
Deep within protons and neutrons lies an even smaller world. Quarks, the fundamental particles of which protons and neutrons are composed, are held together by a powerful force known as the strong nuclear force or binding energy. This force, far stronger than the electrical force, is responsible for the nucleus’s stability despite the repulsive forces between its positively charged protons.
Exploring the Nucleus’s Unique Shape: A Story of Nuclear Deformations
Paragraph 1:
Imagine the nucleus of an atom as a tiny, dense sphere, like a miniature planet at the heart of the atomic realm. In most atoms, this nucleus maintains a perfect spherical shape, a testament to the harmony of nuclear forces. However, in some extraordinary atoms, these forces can conspire to cause fascinating deformations in the nucleus, distorting its shape from perfect symmetry.
Paragraph 2:
These nuclear deformations can range from subtle bulges to dramatic pear-like shapes. The driving force behind these distortions lies in the delicate balance between the repulsive electrostatic forces between protons within the nucleus and the attractive nuclear forces that hold them together. In certain nuclei, the balance tips in favor of repulsion, causing the nucleus to stretch and deform.
Paragraph 3:
The consequences of nuclear deformations are profound. They can alter the energy levels of the nucleus, making certain nuclear reactions more likely or less likely to occur. These deformations can also affect the stability of the nucleus, influencing its susceptibility to radioactive decay. In some cases, extreme deformations can lead to the formation of exotic nuclear shapes, such as the “superdeformed” nuclei found in certain heavy elements.
Paragraph 4:
These nuclear deformations provide a fascinating glimpse into the intricate workings of the atomic nucleus. They demonstrate that even in the subatomic world, where quantum mechanics reigns supreme, there is a delicate balance of forces that determine the structure and behavior of matter. As we delve deeper into the mysteries of the nucleus, we uncover not only its fundamental nature but also the profound influence it has on the world around us.