Nucleus: Atomic Core’s Protons And Neutrons Unveiled
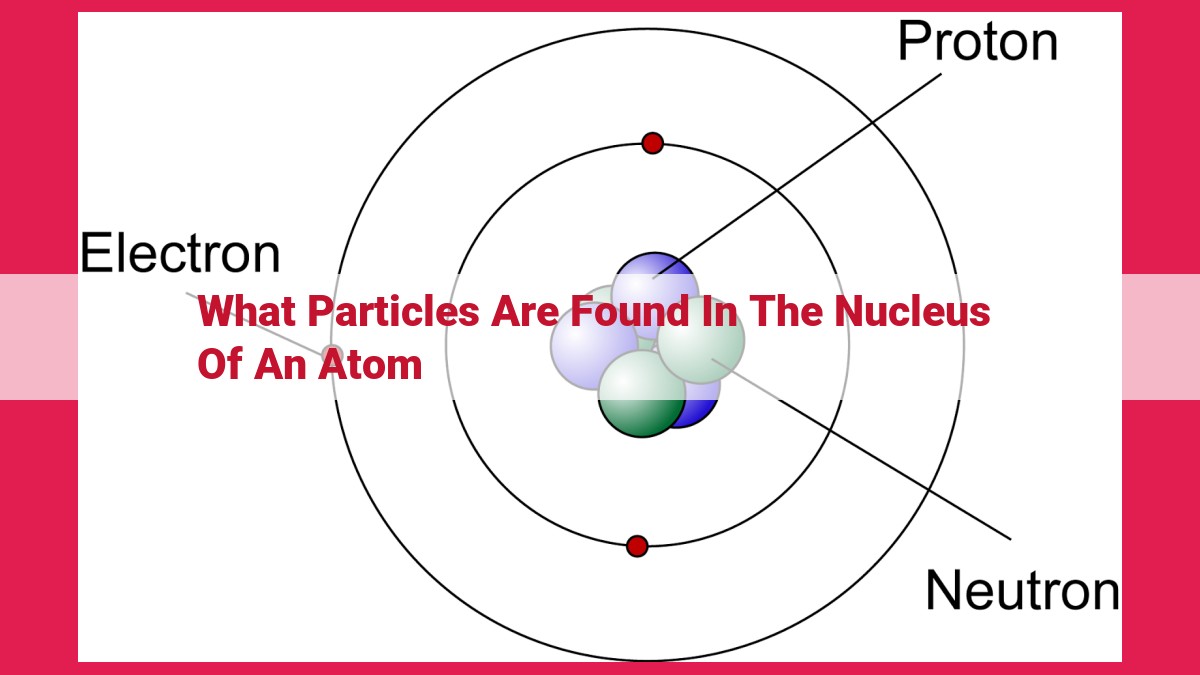
The nucleus of an atom, a central core, harbors two types of particles: protons and neutrons. Protons, with a positive charge, define an element and influence its chemical properties. On the other hand, neutrons, lacking an electric charge, contribute to the mass and stability of an atom. Together, protons and neutrons form nucleons, the building blocks of the nucleus.
- Discuss the focus of the article: exploring the particles found within the atomic nucleus.
- Highlight the importance of understanding nuclear structure in various scientific fields.
Into the Heart of Matter: Unveiling the Particles of the Atomic Nucleus
The world of physics is a mesmerizing realm, where the smallest components of our universe hold immense significance. In the heart of every atom lies the nucleus, a tiny, yet profoundly important region that governs the atom’s identity and behavior. Within this enigmatic core reside a trio of fundamental particles: protons, neutrons, and nucleons. Embark on a captivating journey as we delve into the depths of the atomic nucleus, unraveling the secrets hidden within its confines.
Probing the Enigma: Protons and the Essence of Identity
At the nucleus’s core dwell protons, the positively charged particles that define an atom’s identity. The very essence of an element stems from the number of protons within its nucleus, a property known as the atomic number. Each proton carries a singular positive charge, shaping the atom’s electrical character and dictating its interactions with other elements.
Neutrons: The Unseen Force of Stability
Alongside protons reside neutrons, the mysterious particles that lack an electrical charge. These neutral entities, however, play a crucial role in maintaining the stability of the nucleus. By balancing the opposing forces of protons, neutrons prevent the nucleus from disintegrating into a chaotic soup of charged particles.
The Nucleus: A Dense Hub of Energy and Information
The nucleus stands as the central hub of an atom, a compact yet enormously dense region where protons and neutrons coexist in a delicate equilibrium. Despite its minuscule size, the nucleus captivates with its profound positive charge and harbors a wealth of information regarding an atom’s identity, behavior, and energetic properties.
Protons: The Positively Charged Heart of the Atom
In the heart of every atom, lies a bustling metropolis of subatomic particles, each playing a crucial role in the atom’s overall structure and function. Among these tiny residents, protons stand out as the pillars of the atomic nucleus, carrying a positive charge that defines the atom’s identity.
Protons reside in the very center of the atom, tightly bound together within a compact region known as the nucleus. Along with neutrons, protons constitute the fundamental building blocks of the nucleus, collectively referred to as nucleons. The number of protons in an atom’s nucleus is a defining characteristic, known as the atomic number, which uniquely identifies the element to which the atom belongs.
Protons wield a powerful positive charge that is counterbalanced by the negative charge of electrons orbiting the nucleus. This delicate balance of charges keeps the atom electrically neutral and determines its chemical reactivity. Protons are essential for forming chemical bonds, which hold atoms together to create molecules and compounds that make up everything in the world around us.
Neutrons: The Unsung Heroes of Atomic Stability
Nestled within the heart of every atom lies a tiny universe bustling with subatomic particles. Among these, neutrons stand out as the unsung heroes, contributing silently yet decisively to the stability and identity of all matter.
Unlike their positively charged counterparts, protons, neutrons possess an electrical neutrality that makes them elusive but indispensable. They reside alongside protons within the atomic nucleus, the central core that holds together the fundamental building blocks of our world.
Neutrons play a crucial role in determining an atom’s mass. Each neutron, being slightly heavier than a proton, adds to the overall mass of the atom. This mass number distinguishes isotopes of the same element, which share the same number of protons but differ in their neutron count.
The existence of neutrons is essential for understanding the stability of atoms. Without them, the intense electrostatic repulsion between protons would tear the nucleus apart. Neutrons act as the mediators, neutralizing the positive charge of protons and creating a harmonious balance within the nucleus.
In the realm of chemistry, neutrons influence the behavior of isotopes. Isotopes of the same element have identical chemical properties, as their electronic configurations are identical. However, their different masses can impact their physical properties, such as density and reactivity. This phenomenon finds applications in various fields, including medicine and nuclear energy.
Neutrons not only contribute to the stability and identity of atoms but also play a crucial role in nuclear reactions. They can be easily absorbed or emitted by the nucleus, leading to the formation of new elements or the release of energy. This understanding underpins the principles behind nuclear power and nuclear medicine, offering immense potential for scientific advancement and societal benefits.
Delving into the Heart of the Atom: Unveiling the Secrets of the Nucleus
Within the depths of every atom, lies a tiny universe known as the nucleus. This central region holds the key to understanding the fundamental building blocks of matter.
The nucleus is a bustling hub of activity, teeming with two types of subatomic particles: protons and neutrons. Protons, bearing a positive charge, occupy the nucleus’s core, while neutrons, as their name suggests, carry a neutral charge. These particles, collectively known as nucleons, are the cornerstone of an atom’s identity and stability.
The nucleus is not merely a passive observer. It holds a powerful positive charge, which attracts electrons to the atom’s outer regions. This charge also generates an invisible force field that binds protons and neutrons together, overcoming the natural repulsion of their positive charges.
The size of the nucleus is remarkably small, measuring only about 10^-15 meters in diameter. Despite its diminutive stature, the nucleus packs an incredible amount of mass, accounting for nearly all of an atom’s weight. This density is so great that a single teaspoon of nuclear material would weigh billions of tons.
Understanding the nucleus is crucial for various scientific fields, including nuclear physics, chemistry, and medicine. By unraveling the mysteries of the nucleus, we gain insights into the behavior of matter, the origin of elements, and the potential for nuclear energy.
Atomic Number: Unveiling the Elemental Identity
In our quest to unravel the intricacies of the universe, understanding the structure of atoms is of paramount importance. The atomic nucleus, the bustling core of an atom, harbors fundamental particles that define its unique character and properties. Among these particles, the atomic number stands as a pivotal concept, offering a glimpse into the element to which an atom belongs.
The atomic number is defined as the total number of protons found within the nucleus of an atom. Each element in the periodic table is distinguished by its unique atomic number, which serves as a fingerprint, revealing its chemical identity. For instance, all atoms with an atomic number of 1 are hydrogen, regardless of the number of neutrons or electrons they may possess.
The number of protons within the nucleus is not merely a passive observation; it actively determines the element’s chemical properties and behavior. Protons carry a fundamental positive electric charge, balancing the negative charge of electrons in the surrounding electron cloud. This balance ensures electrical neutrality, a crucial factor in the formation of chemical bonds and the interactions between atoms.
By understanding the atomic number of an element, scientists gain valuable insights into its chemical reactivity, physical characteristics, and potential applications. It allows them to predict the behavior of elements in chemical reactions, design new materials with tailored properties, and comprehend the fundamental interactions that govern the world around us.
In essence, the atomic number serves as a compass, guiding us through the vast expanse of chemical elements. It unlocks the secrets of their nature, enabling us to harness their power and create transformative technologies that shape our modern world.
Mass Number: Distinguishing Isotopes
The mass number of an atom is a crucial concept in understanding the structure of the atomic nucleus. It refers to the total number of protons and neutrons present within the nucleus. Each proton contributes a positive charge of +1, while neutrons are neutral.
The mass number is significant because it distinguishes between isotopes of the same element. Isotopes are atoms that have the same number of protons but differing numbers of neutrons. As a result, they share identical chemical properties but differ in their mass.
For instance, consider the element carbon. Carbon has six protons, giving it an atomic number of 6. The most common isotope of carbon, carbon-12, has an equal number of protons and neutrons, resulting in a mass number of 12. However, other isotopes, such as carbon-13 and carbon-14, have an additional neutron or two, giving them mass numbers of 13 and 14, respectively.
The mass number is a key factor in determining the stability and behavior of an atom. Isotopes with different mass numbers can exhibit different radioactive properties and decay rates. Understanding mass number is essential in fields such as nuclear chemistry, particle physics, and even medicine, where it aids in diagnosing and treating diseases using techniques like isotope imaging.
Isotopes: The Variants of Elements
Within the vast realm of atoms, there exist fascinating variations known as isotopes. Isotopes are atoms of the same element that share an identical number of protons, the positively charged particles within the nucleus. However, they differ in their number of neutrons, the neutral particles also found in the nucleus.
This variation in neutron count results in isotopes having different masses. While isotopes share the same chemical properties due to their identical number of protons and electrons, their mass numbers, the sum of protons and neutrons, vary. This difference in mass is what sets isotopes apart.
For example, the element carbon has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. Carbon-12 contains six protons and six neutrons, while carbon-13 has six protons and seven neutrons. Carbon-14, a radioactive isotope, has six protons and eight neutrons.
The distinction between isotopes has significant implications in various scientific fields. In medicine, radioactive isotopes are used for medical imaging and therapy. For example, iodine-131 is used to treat thyroid disorders and diagnose thyroid cancer. In geology, isotopes are used to determine the age of rocks and fossils, providing valuable insights into Earth’s history.
Understanding isotopes is crucial for unraveling the complexities of the atomic nucleus and its role in shaping the world around us. These atomic variants play a vital role in many scientific disciplines, offering a deeper understanding of the fundamental building blocks of matter.
Nucleons: The Building Blocks of the Atomic Nucleus
Every atom, the fundamental unit of matter, comprises a nucleus at its core, a tiny, dense region where the protons and neutrons, collectively known as nucleons, reside. These subatomic particles determine the fundamental properties of an atom, including its mass and stability.
The mass of an atom is predominantly attributed to its nucleons. Protons, carrying a positive electric charge, and neutrons, electrically neutral, combine within the nucleus to form the core of the atom. Atomic mass, a value used to distinguish elements, is determined by the total number of nucleons present in an atom’s nucleus.
The number of protons within the nucleus defines an element’s atomic number. It is a unique identifier for each element, determining its chemical properties and distinguishing it from all others. Elements with varying numbers of neutrons but identical numbers of protons are known as isotopes.
Isotopes share identical chemical behavior but possess distinct masses due to their differing neutron content. This property has significant implications in various scientific fields, particularly in medicine and nuclear energy. For instance, isotopes of uranium are used as fuel in nuclear reactors, while isotopes of carbon are used in medical imaging techniques.
In summary, nucleons, the collective term for protons and neutrons, play a pivotal role in determining the mass and stability of an atom. Their presence within the atomic nucleus dictates an element’s atomic number, isotopic composition, and ultimately its chemical and physical properties. Understanding the structure and behavior of nucleons is crucial for advancements in various scientific disciplines, ranging from nuclear physics to medicine.