Unveiling The Inert Nature Of Noble Gases: A Comprehensive Guide To Group 18 Elements
Noble gases, located in Group 18 of the periodic table, are renowned for their stability and low reactivity. This is attributed to their complete valence shells, which confer the maximum number of valence electrons possible. Noble gases typically follow the octet rule, possessing eight valence electrons (except helium, which has two). The complete valence shell eliminates the need for these elements to gain or lose electrons, rendering them inert and unreactive.
Valence Electrons: The Key Players in Chemistry’s Dance
Imagine the valence electrons of atoms as tiny dancers, elegantly waltzing around the nucleus, ready to engage in the mesmerizing world of chemical bonding. They are the gatekeepers of reactivity, dictating how elements interact and shaping the vast chemistry we see around us.
The number of valence electrons an element possesses is directly linked to its chemical personality. Elements with fewer valence electrons are eager to form bonds, like shy dancers looking for a partner. They tend to be more reactive, like spirited salsa dancers, giving or taking electrons to achieve stability.
On the other hand, elements with more valence electrons are more aloof, like elegant ballgown dancers. They tend to be less reactive, preferring to keep their electrons to themselves.
Noble Gases: The Unrestrained Elegance of Stability
Amidst the lively chemical dance, the noble gases stand out as enigmatic figures. They possess a full set of valence electrons, creating a stable electronic configuration that makes them remarkably unreactive. Picture them as graceful ballet dancers, their movements so polished and precise that they glide through the world of chemistry without a ripple.
Their complete valence shell fosters an equilibrium that shields them from the need to engage in chemical bonding. They are the paragons of stability, true masters of their own electronic waltz.
Noble Gases: Guardians of Stability
In the captivating realm of chemistry, valence electrons play a pivotal role in determining the behavior of elements. Noble gases, also known as inert gases, stand out as unique entities with extraordinary properties that stem from their distinctive electron configurations.
Identifying the Noble Guardians
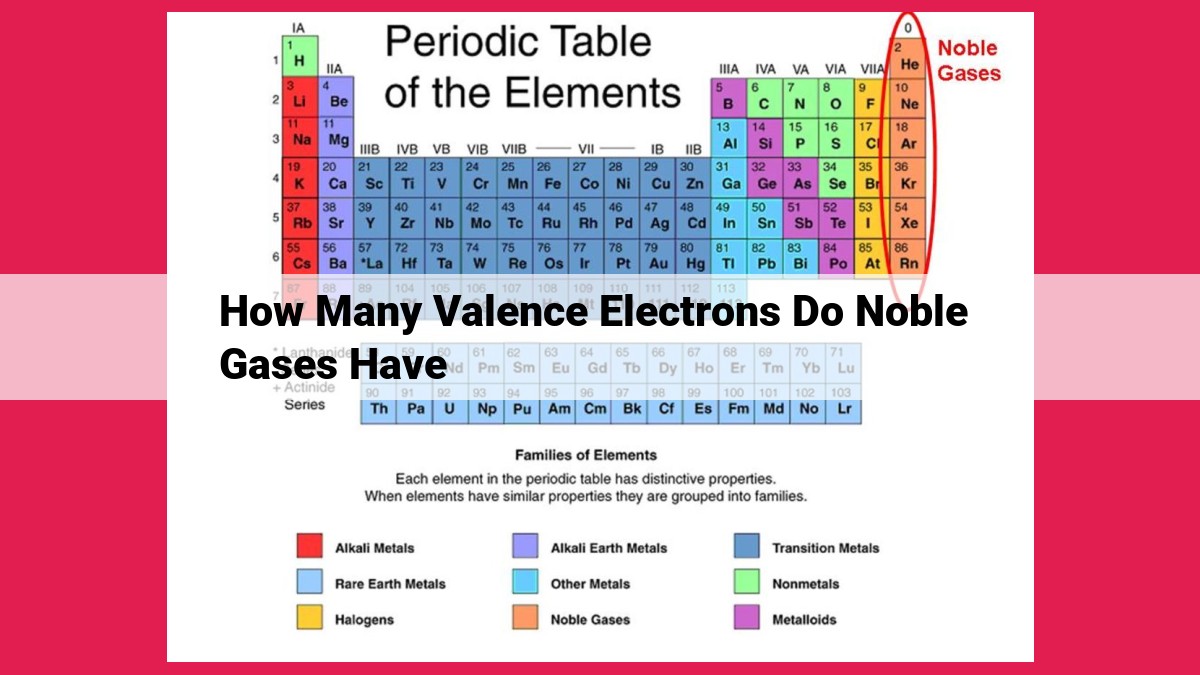
Noble gases occupy a privileged position in the periodic table, nestled in Group 18. They include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). These elements are revered for their exceptional stability and lack of reactivity.
The Electron Configuration Enigma
The secret to the noble gases’ inertness lies in their electron configuration. Unlike other elements, noble gases possess a complete set of valence electrons, giving them a full outer electron shell. This complete shell provides maximum stability, making them reluctant to participate in chemical reactions.
Inertness: A Virtue of Stability
The complete valence shells of noble gases render them chemically inert. They have no desire to gain or lose electrons, ensuring their unwavering stability. This inertness makes them ideal for use in a variety of applications, such as lighting (e.g., neon lights) and diving (e.g., helium in scuba tanks).
Valence Electrons in Noble Gases: The Key to Stability
- State that noble gases have a complete valence shell, providing them with the maximum number of valence electrons possible.
- Explain that this complete valence shell makes them unreactive because there is no need to gain or lose electrons.
- Highlight the octet rule (except for helium) as the typical number of valence electrons for noble gases.
Valence Electrons in Noble Gases: The Secret to Their Unwavering Stability
Picture this: the year is 1904. Sir William Ramsay, a British chemist, makes a groundbreaking discovery. He isolates a colorless, odorless gas that defies all known chemical reactions. This enigmatic element, which he names xenon, becomes the first of the noble gases to be identified.
For years, scientists puzzle over the valence electrons of these peculiar elements. Valence electrons are the outermost electrons in an atom’s electron cloud, and they play a crucial role in chemical bonding. In most elements, valence electrons either orbit alone or share their orbits with neighboring atoms. But in noble gases, something extraordinary happens.
The Magic of a Complete Valence Shell
Unlike other elements, noble gases have a complete valence shell. This means that their valence electrons are arranged in a stable configuration, typically with eight electrons (except for helium, which has two). This complete valence shell creates an incredibly stable electron structure that makes noble gases inert, or unreactive.
Imagine a group of teenagers who have formed a close-knit social circle. They are content with their group and have no desire to add or remove any members. In the same way, noble gases, with their complete valence shells, are completely satisfied with their electron configuration. They have no need to gain or lose electrons, so they remain chemically inert.
The Octet Rule: The Key to Stability
For most noble gases, the octet rule holds true. The octet rule states that atoms are most stable when they have eight valence electrons. This is why noble gases, with their complete valence shells, are so stable. They have achieved the octet configuration, which is the most stable electron arrangement possible.
Noble gases serve as a testament to the importance of valence electrons in determining an element’s reactivity. Elements with incomplete valence shells tend to be more reactive, either because they can gain electrons to complete their valence shells or because they can donate or share electrons to achieve stability. But noble gases, with their complete and stable valence shells, stand as exceptions to this rule, embodying the epitome of chemical stability.
Electron Configuration and Reactivity: A Tale of Two Extremes
In the realm of chemistry, the concept of valence electrons plays a pivotal role in determining the reactivity of elements. Valence electrons are the outermost electrons in an atom’s electron cloud, and their number and arrangement dictate the element’s chemical behavior.
At one extreme, we have noble gases (inert gases), which possess a unique electron configuration that renders them exceptionally stable and unreactive. These elements, such as helium, neon, and argon, reside in the far right-hand column of the periodic table and possess a complete valence shell. This means that their valence shell contains the maximum number of electrons possible, usually eight (except for helium, which has only two).
This complete valence shell configuration creates a stable electron structure, with all electrons paired and no vacancies. As a result, noble gases have very low reactivity because there is no driving force for them to gain or lose electrons. They are content with their electronic arrangement and do not readily participate in chemical reactions.
In contrast, elements with fewer valence electrons than noble gases are more reactive. These elements, such as sodium and chlorine, have valence shells that are not complete and therefore have a tendency to gain or lose electrons to achieve stability. For instance, sodium has one valence electron, which it readily gives up to form positive ions, while chlorine has seven valence electrons and tends to gain an electron to form negative ions.
On the other end of the spectrum, elements with more valence electrons than noble gases are also reactive. These elements, such as oxygen and sulfur, have valence shells that are more than half-filled and therefore have a tendency to donate or share electrons to achieve an octet (eight valence electrons). For example, oxygen has six valence electrons and typically shares two of them with other atoms to form covalent bonds.
In summary, the electron configuration of an element influences its reactivity significantly. Noble gases, with their complete valence shells, are exceptionally stable and unreactive, while elements with incomplete valence shells tend to be more reactive. Understanding this concept is crucial for comprehending the chemical behavior of elements and their ability to form compounds.