Nitrogenous Bases: Essential Building Blocks And Stabilizers Of Genetic Information
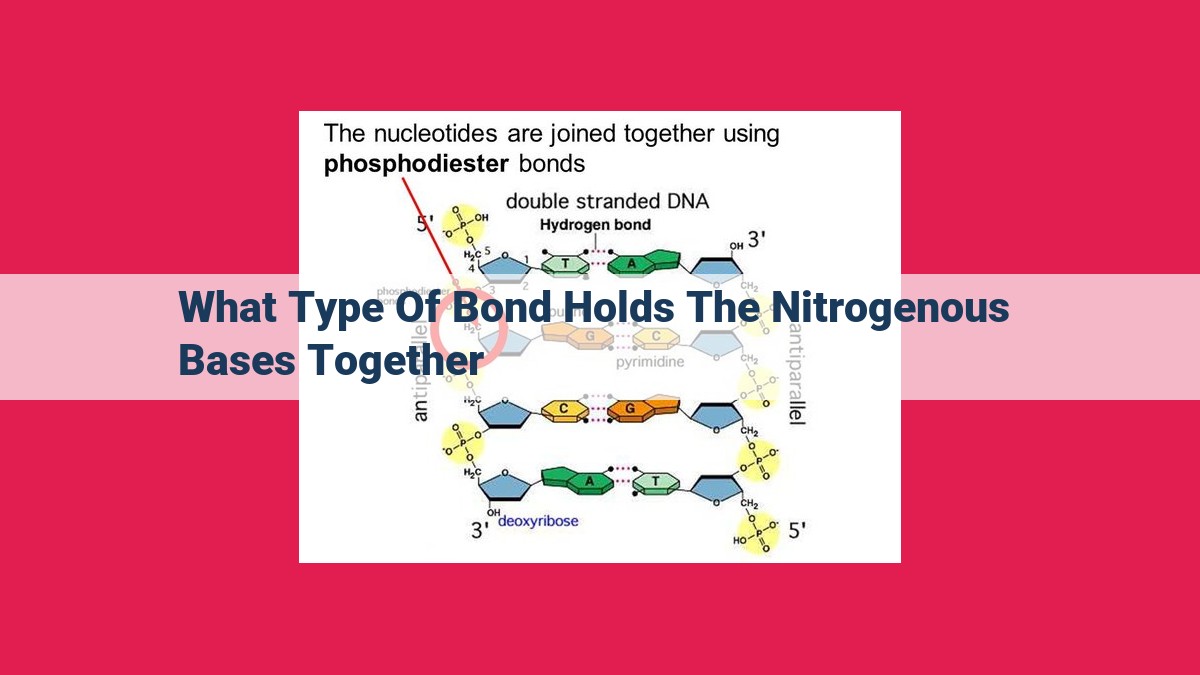
Nitrogenous bases are the building blocks of DNA and RNA, carrying genetic information. These bases are held together by hydrogen bonding, a dipole-dipole interaction where hydrogen atoms share electrons between electronegative atoms. Base pairs form specifically through hydrogen bonding: adenine-thymine (DNA), adenine-uracil (RNA), and guanine-cytosine (DNA/RNA). Hydrogen bonding is the strongest intermolecular force that stabilizes these base pairs, ensuring the structural integrity and genetic function of DNA and RNA.
The Secrets of Life: Unraveling the Importance of Nitrogenous Bases
In the intricate tapestry of life, the microscopic world holds profound secrets that govern our very existence. Nitrogenous bases, the building blocks of DNA and RNA, play a pivotal role in the storage and transmission of genetic information. They orchestrate the traits we inherit and the resilience of our biological systems.
Nitrogenous bases are organic compounds that contain nitrogen atoms and are classified into two main groups: purines (adenine and guanine) and pyrimidines (cytosine, thymine, and uracil). These bases are the alphabet of life, each representing a specific letter in the genetic code. DNA and RNA, the molecules responsible for carrying genetic instructions, are long chains of these nitrogenous bases.
The sequence of nitrogenous bases in DNA and RNA determines the traits and functions of an organism. They act as blueprints, providing the necessary information for the production of proteins and the regulation of cellular processes. Without nitrogenous bases, the transmission of genetic information from one generation to the next would be impossible, relegating life to a perpetual cycle of random mutations.
Hydrogen Bonding: The Key Player in Nitrogenous Base Pairing
In the intricate realm of life’s blueprints, nitrogenous bases serve as the elemental building blocks of DNA and RNA, holding within them the secrets of our genetic heritage. Hydrogen bonding, like an invisible architect, orchestrates the dance of these bases, forging the strong bonds that underpin the very fabric of genetic information.
Hydrogen bonding is a remarkable dipole-dipole interaction that arises when a hydrogen atom is covalently bonded to a highly electronegative atom like nitrogen or oxygen. This unequal sharing of electrons creates a partial positive charge on the hydrogen atom and a partial negative charge on the neighboring electronegative atom. It’s this polarity that allows hydrogen bonding to form between the partial charges of different molecules.
In the context of nitrogenous bases, hydrogen bonding plays a pivotal role in stabilizing the base pairs that determine the genetic code. Adenine (A) forms a double hydrogen bond with thymine (T) in DNA, while in RNA, adenine pairs with uracil (U). Guanine (G) and cytosine (C) form three hydrogen bonds in both DNA and RNA.
These specific hydrogen bonding patterns are crucial for maintaining the stability and integrity of the DNA double helix. The antiparallel arrangement of DNA strands, with the sugar-phosphate backbone facing outward and the nitrogenous bases facing inward, is stabilized by hydrogen bonding between complementary base pairs.
Furthermore, hydrogen bonding is not just a passive bystander in genetic processes. Its dynamic nature allows for the breaking and reforming of base pairs during DNA replication and transcription. This plasticity enables the precise copying and expression of genetic information, ensuring the faithful transmission of genetic traits from generation to generation.
In summary, hydrogen bonding is the unsung hero of nitrogenous base pairing, the force that orchestrates the molecular interactions that underpin the stability and function of DNA and RNA. Without hydrogen bonding, the intricate tapestry of life’s genetic makeup would unravel, leaving us lost in a genetic void.
Nitrogenous Base Pairing: A Match Made in Bonding
In the molecular realm of DNA and RNA, the intricate arrangement of nitrogenous bases lay the foundation for the genetic blueprint of life. These nitrogenous bases, the building blocks of our genetic heritage, are held together by a dance of intermolecular forces, with hydrogen bonding taking center stage.
The four nitrogenous bases are adenine (A), thymine (T), guanine (G), and cytosine (C). In the double helix of DNA, adenine pairs exclusively with thymine, while guanine forms a strong bond with cytosine. This specific pairing, dictated by the hydrogen bonds between these bases, creates the iconic ladder-like structure of DNA.
In RNA, a single-stranded cousin of DNA, adenine pairs with uracil (U) instead of thymine. This subtle change, while maintaining the hydrogen bonding pattern, gives RNA its unique properties and functions.
The specificity of hydrogen bonding in base pairing is crucial for the stability and accuracy of genetic information. The strong dipole-dipole interactions between hydrogen atoms and electronegative nitrogen and oxygen atoms form stable base pairs. This structural integrity ensures that genetic information can be accurately replicated, transcribed, and translated into proteins, the essential building blocks of life.
Key Points to Remember:
- Nitrogenous bases are the building blocks of DNA and RNA.
- Hydrogen bonding is the primary force that holds together nitrogenous bases in specific pairs:
- Adenine-Thymine (DNA)
- Adenine-Uracil (RNA)
- Guanine-Cytosine (DNA and RNA)
- The specificity of hydrogen bonding ensures the stability and accuracy of genetic information.
Intermolecular Forces: The Supporting Cast in Nitrogenous Base Pairing
Intermolecular forces are the non-covalent interactions that occur between molecules. These forces are responsible for holding molecules together in a condensed state (such as liquids and solids) and play a crucial role in numerous biological processes, including nitrogenous base pairing.
There are various types of intermolecular forces, each with its own strength and characteristics:
- Hydrogen bonding: This is the最強 intermolecular force and arises from the attraction between a hydrogen atom covalently bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom. Hydrogen bonding is particularly important in nitrogenous base pairing, where it forms between specific base pairs (adenine-thymine in DNA and adenine-uracil in RNA, as well as guanine-cytosine in both DNA and RNA).
- Dipole-dipole interactions: These occur between molecules that have permanent dipole moments, which means they have a positive end and a negative end. Dipole-dipole interactions are typically weaker than hydrogen bonding but can contribute to the stability of certain molecular arrangements.
- Van der Waals forces: These are weak interactions that include dispersion forces (instantaneous attractions between fluctuating electron clouds) and permanent dipole-induced dipole interactions. Van der Waals forces are the weakest type of intermolecular force and typically contribute only slightly to the stability of molecular assemblies.
In the context of nitrogenous base pairing, hydrogen bonding is the dominant intermolecular force that stabilizes the base pairs. The strong dipole-dipole interactions between the hydrogen bond donors and acceptors in the base pairs create a highly directional and specific interaction that is essential for the stability and correct functioning of DNA and RNA.
Hydrogen Bonding’s Superiority in Nitrogenous Base Pairing
In the realm of genetics, nitrogenous bases are the building blocks of DNA and RNA, the molecules that carry our genetic information. These bases pair up through hydrogen bonding, a special type of dipole-dipole interaction. Hydrogen bonding is the unsung hero of genetics, stabilizing base pairs and ensuring the integrity of our genetic code.
Hydrogen bonding is a relatively strong intermolecular force that arises when a hydrogen atom is bonded to a highly electronegative atom, such as nitrogen or oxygen. This creates a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom. The partial positive charge of one hydrogen atom is attracted to the partial negative charge of another electronegative atom, forming a hydrogen bond.
In the context of nitrogenous bases, hydrogen bonding plays a crucial role in maintaining the double helix structure of DNA. Adenine and thymine, as well as guanine and cytosine, pair up specifically through hydrogen bonding. Adenine and thymine form two hydrogen bonds, while guanine and cytosine form three hydrogen bonds, which increases the stability of the base pairs.
The strength of hydrogen bonding in nitrogenous base pairing is essential for the stability of DNA. The hydrogen bonds hold the base pairs together, preventing the double helix from unwinding or breaking apart. This stability is crucial for the accurate transmission of genetic information during cell division and for the proper functioning of genes.
Without hydrogen bonding, nitrogenous bases would not be able to pair up specifically and form the double helix structure of DNA. This would have disastrous consequences for life, as DNA is the blueprint for all living organisms. Hydrogen bonding is the foundation of genetic information, ensuring the stability and integrity of our genetic code.