Nitrogen Cycle: Essential For Life And Environmental Balance
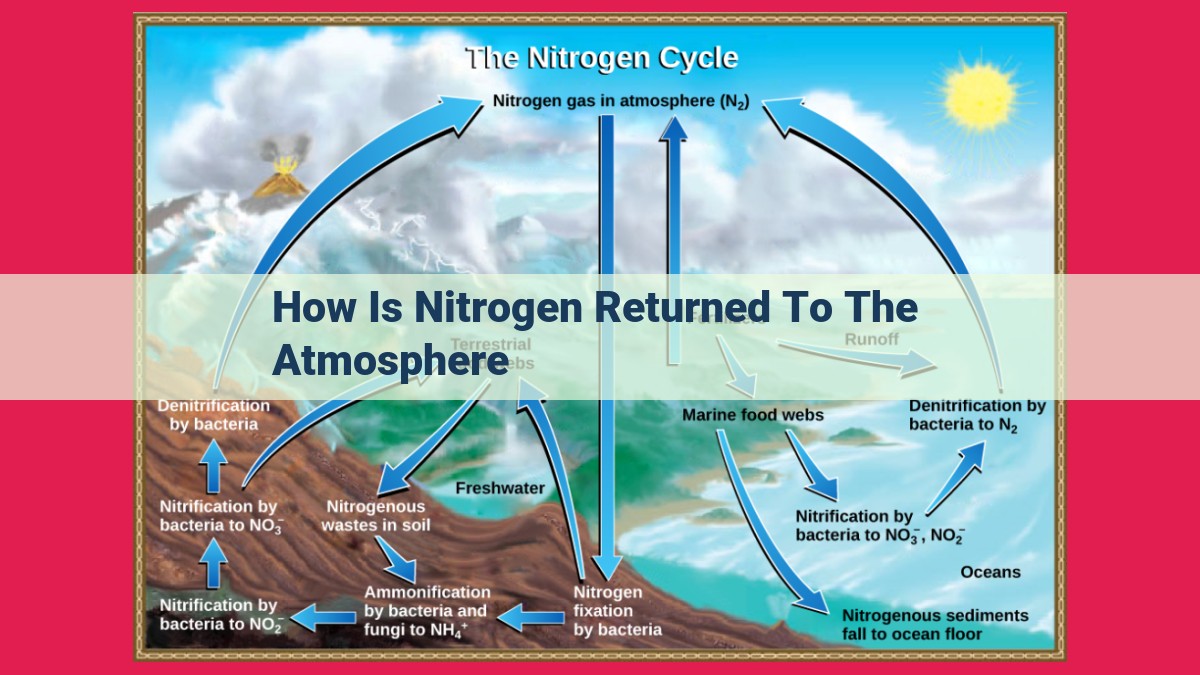
Nitrogen, essential for life, is returned to the atmosphere through denitrification, a process in which nitrates are converted back into nitrogen gas by bacteria. Nitrogen-fixing bacteria convert inert atmospheric nitrogen into ammonia, which is then oxidized into nitrites and nitrates. These compounds can be used by plants or undergo denitrification. Urea, another nitrogen-containing compound, is broken down by urease, releasing ammonia for further cycling.
The Vital Role of Nitrogen: The Building Block of Life
Nitrogen, an indispensable element, plays a pivotal role in sustaining life on Earth. It constitutes a significant proportion of proteins, nucleic acids, and chlorophyll. These essential molecules are responsible for protein synthesis, genetic inheritance, and photosynthesis, providing the foundation for all living organisms.
Without nitrogen, the delicate balance of life would be disrupted. Proteins are the workhorses of the cell, carrying out countless biochemical reactions. Nucleic acids, such as DNA and RNA, hold the blueprints for life, ensuring genetic continuity. Chlorophyll captures sunlight during photosynthesis, converting it into energy that fuels plant growth and provides the basis for the food chain.
Nitrogen is the most abundant element in the atmosphere; however, it exists in a form that is not directly accessible to most living organisms. To make nitrogen usable, it must undergo a process known as nitrogen fixation.
Subheading: Nitrogen Fixation: Transforming Inert Nitrogen into Usable Forms
Nitrogen fixation is a critical step that converts atmospheric nitrogen into ammonia, a form that can be utilized by plants and other organisms. This transformation is primarily carried out by nitrogen-fixing bacteria that reside in the soil or in symbiotic relationships with plants.
Nitrogen Fixation: The Alchemy of Life
In the tapestry of life, nitrogen holds a special place. It’s the cornerstone of proteins, the building blocks of life, and it plays a crucial role in countless biological processes. Yet, nitrogen in its gaseous form is inert, a stubborn molecule that stubbornly resists chemical reactions. So, how does this essential element become available to living organisms? The answer lies in a remarkable process known as nitrogen fixation.
The atmosphere is a vast reservoir of nitrogen, but it’s simply too stable for most organisms to use. Enter nitrogen-fixing bacteria, the unsung heroes of the microbial world. These tiny organisms possess a unique ability: they can convert atmospheric nitrogen into ammonia, a form that plants and other organisms can readily absorb and utilize.
The process of nitrogen fixation is a delicate dance of chemistry and biology. Within the cells of nitrogen-fixing bacteria, a specialized enzyme called nitrogenase works its magic. Nitrogenase breaks the strong bonds of the nitrogen molecule, allowing the atoms to form ammonia. This transformation is akin to alchemy, turning the inert element into a substance of life.
Nitrogen-fixing bacteria live in a variety of habitats, from the soil to the oceans. Some form symbiotic relationships with plants, such as Rhizobium bacteria that reside in the root nodules of legumes. These partnerships allow plants to access fixed nitrogen in exchange for carbohydrates from the plant. Other nitrogen-fixing bacteria live independently, making their contribution to the global nitrogen cycle.
Nitrogen fixation is a foundational process that supports life on Earth. It provides the basic building material for proteins, nucleic acids, and other essential molecules. Without nitrogen-fixing bacteria, the ecosystem would collapse, and life as we know it would not be possible.
Ammonia Oxidation: Converting Ammonia into Nitrites and Nitrates: Describe the process of ammonia oxidation and the role of bacteria in this transformation.
Ammonia Oxidation: The Vital Transformation in Nitrogen Cycling
In the intricate symphony of life on Earth, nitrogen plays a pivotal role as a fundamental building block of proteins, nucleic acids, and chlorophyll. However, the vast majority of atmospheric nitrogen exists in an inert form, inaccessible to most living organisms.
To make nitrogen usable, a remarkable process called ammonia oxidation occurs. This transformation converts ammonia, a simple compound containing nitrogen and hydrogen, into nitrites and nitrates, molecules that plants and other organisms can readily utilize.
The stage for ammonia oxidation is set by a group of specialized bacteria that possess the enzymatic machinery to catalyze this chemical reaction. These ammonia-oxidizing bacteria reside in soil, water, and even within the roots of certain plants. As ammonia diffuses into their surroundings, these bacteria set to work.
Within their cells, the bacteria employ a complex set of enzymes that orchestrate the oxidation of ammonia. The key player in this process is the ammonia monooxygenase enzyme, which inserts an oxygen atom into the ammonia molecule, producing hydroxylamine. This intermediate compound is then further oxidized to nitrite and subsequently to nitrate.
The production of nitrites and nitrates by ammonia-oxidizing bacteria is crucial for the nutrient cycling in ecosystems. Nitrites and nitrates are assimilated by plants, fueling their growth and productivity. Additionally, these compounds play a role in nitrogen fixation, the process by which inert atmospheric nitrogen is converted into biologically available forms.
The intricate process of ammonia oxidation is a testament to the remarkable abilities of microorganisms to transform and utilize elements essential for life. Without these microbial wizards, the cycling of nitrogen in the biosphere would be severely compromised, jeopardizing the very fabric of the ecosystems we depend on.
Denitrification: Releasing Nitrogen Back into the Atmosphere: Explain how nitrates and nitrites are converted back into atmospheric nitrogen through denitrification, including the bacteria involved in this process.
Denitrification: The Final Chapter in Nitrogen’s Journey
In the intricate symphony of life on Earth, nitrogen plays a pivotal role. But its journey through the ecosystem is far from linear. After being transformed into usable forms, it must eventually return to its gaseous state in the atmosphere. This intricate process, known as denitrification, is the final chapter in nitrogen’s odyssey.
Denitrification is a complex biochemical reaction, facilitated by a select group of bacteria known as denitrifying bacteria. These skilled microorganisms possess the remarkable ability to convert nitrates and nitrites, the oxidized forms of nitrogen, back into atmospheric nitrogen. This intricate process takes place in oxygen-depleted environments, such as waterlogged soils or the depths of oceans.
The journey begins with the reduction of nitrates to nitrites. This delicate operation is carried out by enzymes within denitrifying bacteria, which catalyze the transfer of electrons. Nitrites, now unstable, undergo a further transformation as they lose an oxygen atom and transform into nitric oxide.
This versatile molecule can follow two distinct paths. Under high oxygen conditions, it may react with oxygen to form nitrous oxide, which can contribute to climate change as a potent greenhouse gas, or it can be further reduced to dinitrogen gas, the inert form of nitrogen that returns to the atmosphere.
Denitrifying bacteria play a crucial role in maintaining the nitrogen balance in the ecosystem. By releasing nitrogen back into the atmosphere, they complete the nitrogen cycle, ensuring a continuous supply of this essential element for life on our planet. Their contribution to the nitrogen cycle highlights the intricate and interconnected nature of Earth’s ecosystems and underscores the importance of preserving these delicate processes.
Nitrous Oxide Emission and Climate Change: A Threat to Our Planet
The Invisible Culprit:
Nitrogen is essential for life, but one form of it poses a hidden danger to our planet: nitrous oxide, the third most important greenhouse gas after carbon dioxide and methane. This colorless and odorless gas has a global warming potential nearly 300 times that of carbon dioxide, contributing significantly to the ongoing climate crisis.
The Source of Nitrous Oxide:
Nitrous oxide is produced naturally through microbial processes in soils, oceans, and wetlands. However, human activities have dramatically increased its emissions. The burning of fossil fuels, agricultural practices, and industrial processes release substantial amounts of nitrous oxide into the atmosphere.
The Greenhouse Effect:
Nitrous oxide traps heat in the atmosphere, acting like a blanket around the Earth. This greenhouse effect leads to a rise in global temperatures, causing a myriad of adverse effects, including melting ice caps, rising sea levels, and extreme weather events.
Mitigating Nitrous Oxide Emissions:
Recognizing the severe consequences of nitrous oxide emissions, scientists and policymakers are working to mitigate its impact. This involves:
- Regulating industrial processes: Implementing emission control technologies and adopting best practices to reduce nitrous oxide releases.
- Improving agricultural techniques: Promoting sustainable fertilizer management, reducing nitrogen application rates, and employing cover crops to minimize soil emissions.
- Protecting ecosystems: Restoring wetlands and grasslands that naturally absorb nitrous oxide, while conserving forests that act as carbon sinks.
A Collective Effort:
Addressing nitrous oxide emissions requires a collective effort from governments, industries, and individuals. By reducing our dependence on fossil fuels, adopting sustainable farming practices, and protecting our natural ecosystems, we can mitigate the threat posed by this potent greenhouse gas and secure a more stable climate for future generations.
Urease: The Unsung Hero of Nitrogen Cycling
In the intricate tapestry of life on Earth, nitrogen plays a pivotal role as the building block of proteins, nucleic acids, and many other essential molecules. Nitrogen cycling ensures that this vital element is available to living organisms, and one of the key players in this process is the enzyme urease.
Urease is a specialized enzyme that catalyzes the hydrolysis of urea, a waste product produced by animals and plants. When urea is broken down, it releases ammonia and carbon dioxide. Ammonia is then further oxidized to nitrite and nitrate, which can be used by plants for growth. Carbon dioxide, on the other hand, is released into the atmosphere and participates in the global carbon cycle.
The significance of urease in nitrogen cycling cannot be overstated. Urease facilitates the re-entry of nitrogen into the biosphere, making it available for plants to synthesize proteins and other nitrogen-containing compounds. Without urease, urea would accumulate in the environment, disrupting the delicate balance of nitrogen cycling and potentially leading to nitrogen deficiencies in plants.
Moreover, urease activity is crucial for maintaining soil fertility. Ammonia and nitrates produced by urease hydrolysis promote plant growth, providing essential nutrients for a variety of crops. Farmers often apply urea-based fertilizers to enhance soil fertility and increase crop yields.
Understanding the role of urease in nitrogen cycling helps us appreciate the intricate connections between living organisms and their environment. It also highlights the importance of preserving soil health and reducing activities that disrupt the natural nitrogen cycle. By acknowledging the unsung hero of nitrogen cycling, we can contribute to a more sustainable and productive planet.