Nitrogen: Atomic Number, Electron Configuration, And Valence Electrons
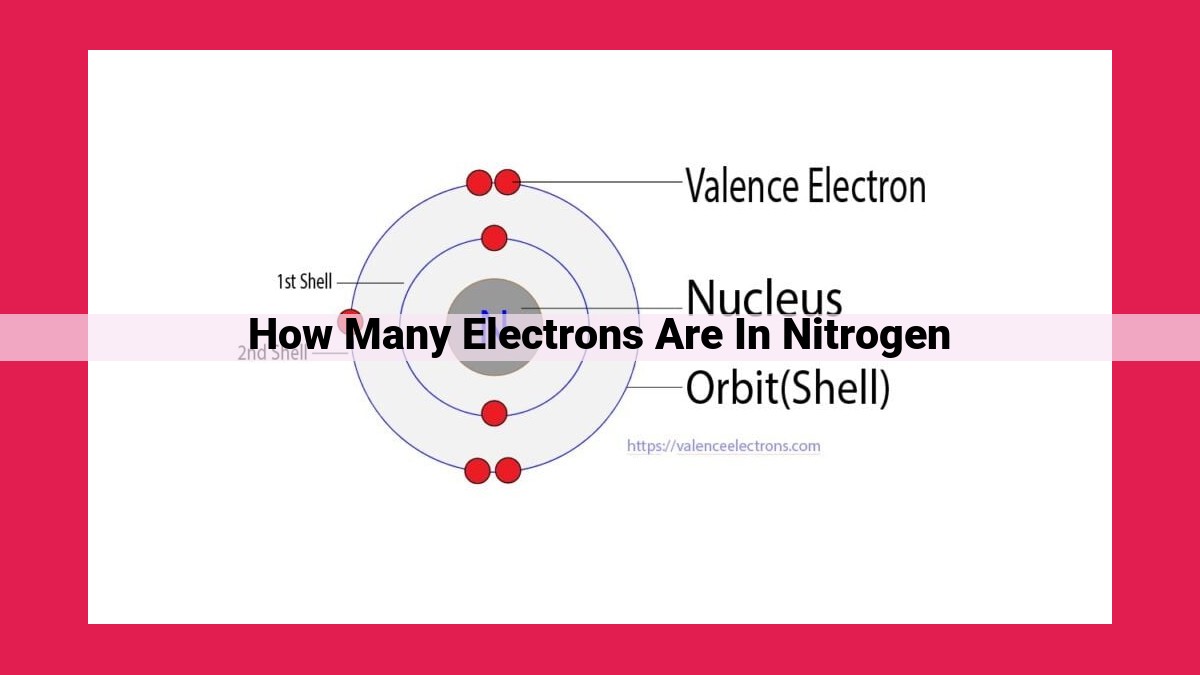
Nitrogen has 7 electrons as its atomic number, which represents the number of protons in its nucleus, is 7. Since neutral atoms have an equal number of protons and electrons, nitrogen must also have 7 electrons. This is further confirmed by its electron configuration: 1s²2s²2p³. The numbers in the electron configuration indicate the number of electrons in each energy level, with the number after the letter “s” or “p” representing the specific orbital. In nitrogen’s case, it has 2 electrons in the 1s orbital, 2 in the 2s orbital, and 3 in the 2p orbital, totaling 7 electrons.
Exploring the Atomic Structure of Nitrogen
Nitrogen, the abundant element that constitutes about 78% of our atmosphere, holds many secrets in its atomic makeup. To unravel these mysteries, let’s delve into the fascinating realm of its atomic structure.
The Building Blocks of Atoms
At its core, an atom is the smallest unit of an element that retains its chemical properties. It consists of a tiny, dense nucleus surrounded by a cloud of even tinier particles called electrons. The nucleus, in turn, is made up of positively charged protons and neutral neutrons.
The Concept of Atomic Number
Each element is uniquely identified by its atomic number, which represents the number of protons in its nucleus. Nitrogen has an atomic number of 7, signifying the presence of 7 protons in its nucleus. The atomic number is of paramount importance as it determines the element’s position in the Periodic Table of Elements.
In summary, the atomic structure of nitrogen comprises 7 protons and a corresponding number of electrons in a neutral atom, giving it an atomic number of 7. Armed with this knowledge, we can continue our exploration into the captivating features of nitrogen’s electron configuration.
Nitrogen: Exploring the Atomic Realm
Nitrogen, an essential element for life on Earth, possesses a fascinating atomic structure that unveils its unique properties.
Unveiling the Atom’s Architecture
Every atom consists of a nucleus, the central core, and a swarm of electrons orbiting around it. The nucleus houses positively charged protons and electrically neutral neutrons. Each element is distinguished by its atomic number, which represents the number of protons in the nucleus.
Electron Configuration: The Energy Ladder
Electrons reside in distinct energy levels around the nucleus. Each energy level can hold a specific number of electrons in various subshells. The electron configuration of an element describes the arrangement of its electrons in these subshells.
For instance, nitrogen’s electron configuration is 1s²2s²2p³, indicating that it has two electrons in the first energy level (1s), two in the second energy level (2s), and three in the third energy level (2p).
Electron Balance: Nature’s Equilibrium
In a neutral atom, the number of electrons is equal to the number of protons. This balance ensures that the atom has no overall electrical charge. Therefore, nitrogen, with an atomic number of 7, possesses 7 electrons in its electron configuration.
Understanding nitrogen’s atomic structure is crucial for comprehending its chemical behavior and reactivity. As an essential part of amino acids and nucleic acids, nitrogen plays a pivotal role in biological processes and the very fabric of life itself.
Delving into the Atomic World of Nitrogen
Unveiling the Essence of Atoms
Every atom, the fundamental building block of matter, comprises a dense nucleus orbited by electrons. At the heart of the nucleus reside protons (positively charged) and neutrons (neutral). These particles collectively determine an atom’s atomic number, a unique identifier that defines its element on the periodic table.
Electron Configuration: Mapping Energy’s Dance
Electrons, negatively charged, arrange themselves in distinct energy levels or shells surrounding the nucleus. Each shell can accommodate a specific number of electrons, with the outermost shell holding the most. The electron configuration of an element describes how its electrons distribute among these levels.
Nitrogen’s Electron Ensemble
Nitrogen, an essential component of life on Earth, boasts an electron configuration of 1s²2s²2p³. This notation indicates that:
- Two electrons occupy the innermost 1s orbital closest to the nucleus.
- Two more reside in the next higher-energy 2s orbital.
- The remaining three electrons inhabit the 2p orbital, arranged in a triangular formation.
Balancing the Atomic Ledger
In a neutral atom, the number of positively charged protons equals the number of negatively charged electrons. Since nitrogen’s atomic number is 7, we deduce that it possesses 7 electrons to maintain electrical neutrality. This harmonious balance is crucial for the stability of any atom.