Nickel’s Valence Electrons: Unlocking Chemical Bonding And Element Interactions
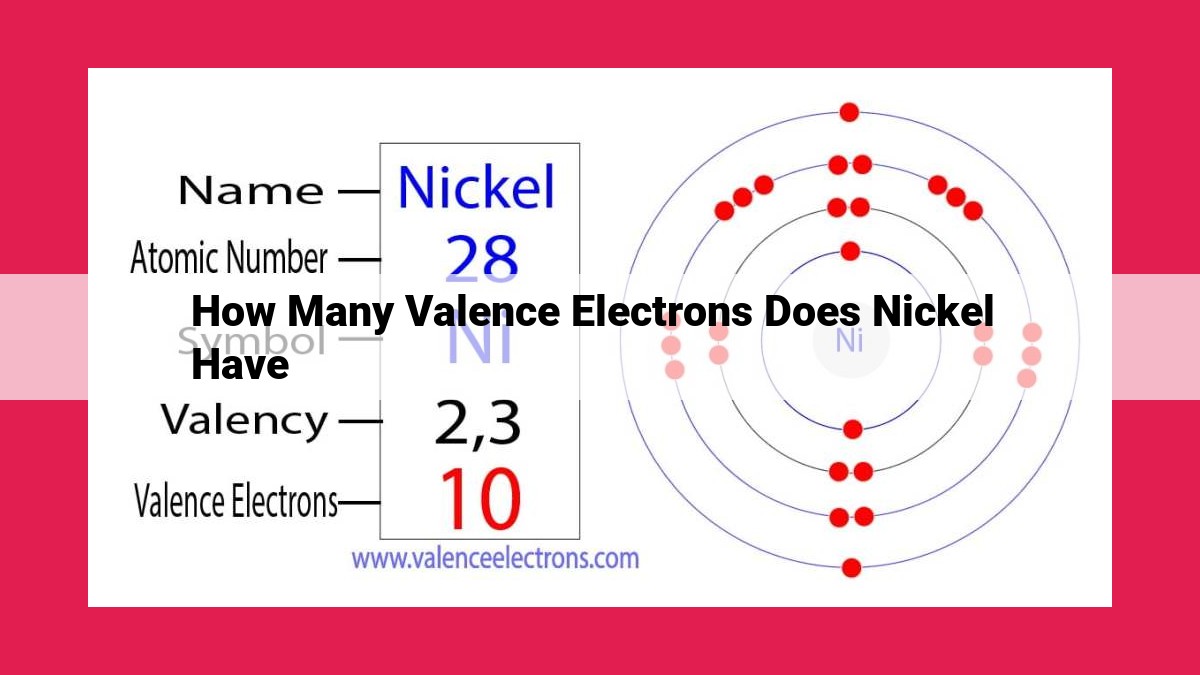
Nickel has 10 valence electrons, which are the electrons in the outermost shell of its atomic structure. Valence electrons are crucial for chemical bonding, as they determine an element’s ability to interact with others. By examining nickel’s electron configuration, we can identify these valence electrons and understand their significance in its chemical properties.
Understanding Valence Electrons: The Key to Chemical Bonding
In the realm of chemistry, electrons dance around atomic nuclei, forming the building blocks of matter. Valence electrons, in particular, hold a pivotal role in these lively interactions. Defined as the electrons occupying the outermost energy level of an atom, they are crucial players in determining the chemical behavior of elements.
Valence electrons are like social butterflies, eager to form bonds with other atoms. They participate in chemical reactions, sharing or exchanging electrons to achieve a stable configuration. This dance of electrons is the driving force behind the formation of molecules, compounds, and all the diverse materials that make up our world.
Imagine a dinner party where valence electrons are the guests. Each atom brings its own set of valence electrons, ready to mingle. These electrons determine the atom’s ability to form connections, much like the outgoing personalities of guests at a social event. The number and arrangement of valence electrons in an atom’s electron configuration govern its chemical properties.
Nickel’s Electron Configuration: Unveiling the Building Blocks of a Versatile Metal
In the realm of chemistry, understanding the electron configuration of elements is akin to deciphering a secret code that unravels their unique properties. For nickel (Ni), a transition metal with remarkable versatility, its electron configuration holds the key to unlocking its chemical behavior.
Nickel’s atomic number is 28, indicating the presence of 28 electrons within its atomic structure. These electrons occupy specific energy levels called orbitals, which are arranged in shells around the nucleus. Nickel’s electron configuration can be written as:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸ 4s²
This notation indicates that the first two electrons occupy the 1s orbital, the next two occupy the 2s orbital, the next six the 2p orbital, and so on, until the outermost four electrons are found in the 3d and 4s orbitals.
The outermost electrons, known as valence electrons, play a crucial role in determining nickel’s chemical properties. These valence electrons are the most loosely bound to the nucleus and are responsible for engaging in chemical bonding with other atoms. In nickel’s case, it possesses two valence electrons in the 4s orbital and eight valence electrons in the 3d orbital, for a total of ten valence electrons.
Understanding Valence Electrons in Nickel’s Chemistry
Have you ever wondered why some elements readily bond with each other while others simply don’t play well together? The key to this chemical dance lies in understanding valence electrons. They are like the social butterflies of the atomic world, determining the interactions between elements.
What are Valence Electrons?
Valence electrons are the electrons that reside in an atom’s outermost energy level. In other words, they are the electrons that are most loosely bound to the nucleus and have the highest energy. These electrons participate in chemical bonding, the process by which atoms combine to form compounds.
Nickel’s Electron Configuration
Nickel is an intriguing element with the atomic number 28. Its electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸ 4s²
This means that nickel has 28 electrons, distributed among its four energy levels. The outermost energy level (4s) contains two valence electrons, as indicated by the bold portion:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸ 4s²
Identifying Valence Electrons
To determine the number of valence electrons in an atom, simply look at the outermost energy level. In the case of nickel, the bold 4s² indicates that it has two valence electrons.
Valence Electrons and the Periodic Table
The position of an element in the periodic table can provide clues about its valence electrons. Elements in the same group (vertical column) typically have the same number of valence electrons. For instance, nickel is in Group 10, which means it has ten valence electrons.
Significance of Valence Electrons
Valence electrons play a pivotal role in nickel’s chemistry, influencing:
- Chemical Bonding Patterns: Valence electrons determine how nickel bonds with other elements. For example, its two valence electrons enable it to form two covalent bonds.
- Formation of Compounds: The number of valence electrons helps predict the types of compounds that nickel can form. Nickel can react with other elements to create compounds such as nickel oxide (NiO) and nickel chloride (NiCl₂).
- Oxidation States: Valence electrons determine nickel’s oxidation states, which represent the number of electrons that it can lose or gain in chemical reactions.
- Reactivity with Other Elements: The number of valence electrons influences nickel’s reactivity with other elements. Nickel is a relatively reactive metal, readily reacting with oxygen, chlorine, and other non-metals.
Chemical Bonding and the Periodic Table: A Dance of Valence Electrons
In the vast expanse of the periodic table, each element dances to its own rhythm, dictated by the valance electrons that reside in its outermost shell. These nimble electrons play a pivotal role in the intricate choreography of chemical bonding, determining an element’s tendency to form bonds and the shape of the molecules it creates.
The periodic table, a masterpiece of chemical organization, arranges elements based on their atomic number, which corresponds to the number of protons in their nucleus. This arrangement reveals fascinating patterns that govern the behavior of valence electrons. Elements in the same group, sharing a vertical column, have the same number of valence electrons, giving them similar chemical properties.
For instance, Group 1 elements, like sodium and potassium, each possess a single valence electron. This shared characteristic makes them highly reactive and eager to form bonds, resulting in their classic “ping-pong” reactions with water. Conversely, Group 18 elements, also known as noble gases, have a complete valence shell, making them chemically stable and reluctant to participate in bonding.
The horizontal rows of the periodic table, or periods, represent the energy levels of electrons. Elements within a period share the same number of energy levels and exhibit varying numbers of valence electrons. This variation influences their chemical bonding preferences and reactivities.
Understanding the relationship between valance electrons and the periodic table empowers chemists to predict the bonding behavior of elements and envision the molecular landscapes they create. It’s like having a secret code that unlocks the mysteries of chemistry, revealing the intricate connections between the elements that shape our world.
The Significance of Valence Electrons in Understanding Nickel’s Chemical Properties
In the realm of chemistry, comprehending the concept of valence electrons is crucial for unlocking the intricacies of an element’s chemical behavior. These electrons, located in the outermost energy level of an atom, play a pivotal role in determining the element’s ability to form chemical bonds and interact with other substances. In this context, nickel emerges as a fascinating element, offering valuable insights into the significance of valence electrons.
As an atomic number of 28, nickel possesses an electron configuration of 1s²2s²2p⁶3s²3p⁶3d⁸4s². This configuration reveals that nickel has eight valence electrons residing in its outermost 3d and 4s orbitals. These valence electrons dictate nickel’s chemical properties and govern its interactions with other elements.
Chemical Bonding Patterns
Valence electrons act as matchmakers in the atomic world, facilitating the formation of chemical bonds between atoms. Nickel’s eight valence electrons enable it to participate in a variety of bonding scenarios. It can form strong metallic bonds with itself, creating a stable and durable metal lattice structure. Moreover, nickel’s valence electrons allow it to form covalent bonds with non-metallic elements, sharing electrons to achieve a stable electron configuration.
Formation of Compounds
The number and arrangement of valence electrons dictate the types of compounds an element can form. Nickel’s eight valence electrons make it a versatile bonding partner. It can form a wide range of compounds, including oxides, sulfides, chlorides, and complexes. These compounds exhibit diverse properties and applications, ranging from catalysis to corrosion resistance.
Oxidation States
Valence electrons also influence an element’s oxidation states, which represent the different ways it can lose or gain electrons during chemical reactions. Nickel can exhibit multiple oxidation states, including +2 and +3. These oxidation states arise from the ability of nickel’s valence electrons to participate in redox reactions, where electrons are transferred between atoms or ions.
Reactivity with Other Elements
The number of valence electrons also affects an element’s reactivity with other elements. Nickel’s eight valence electrons confer a moderate reactivity. It readily reacts with oxygen to form a protective oxide layer, but it is less reactive than other transition metals such as iron or copper. This moderate reactivity makes nickel suitable for various applications, such as electroplating, alloying, and catalysis.
In conclusion, valence electrons play a pivotal role in shaping the chemical properties of nickel. They determine its bonding patterns, influence the formation of compounds, govern its oxidation states, and affect its reactivity with other elements. Understanding the significance of valence electrons provides a deeper comprehension of nickel’s behavior and enables scientists and engineers to harness its unique properties in a wide range of applications.