Understanding The Contribution Of Neutrons To Gold’s Atomic Structure And Practical Applications
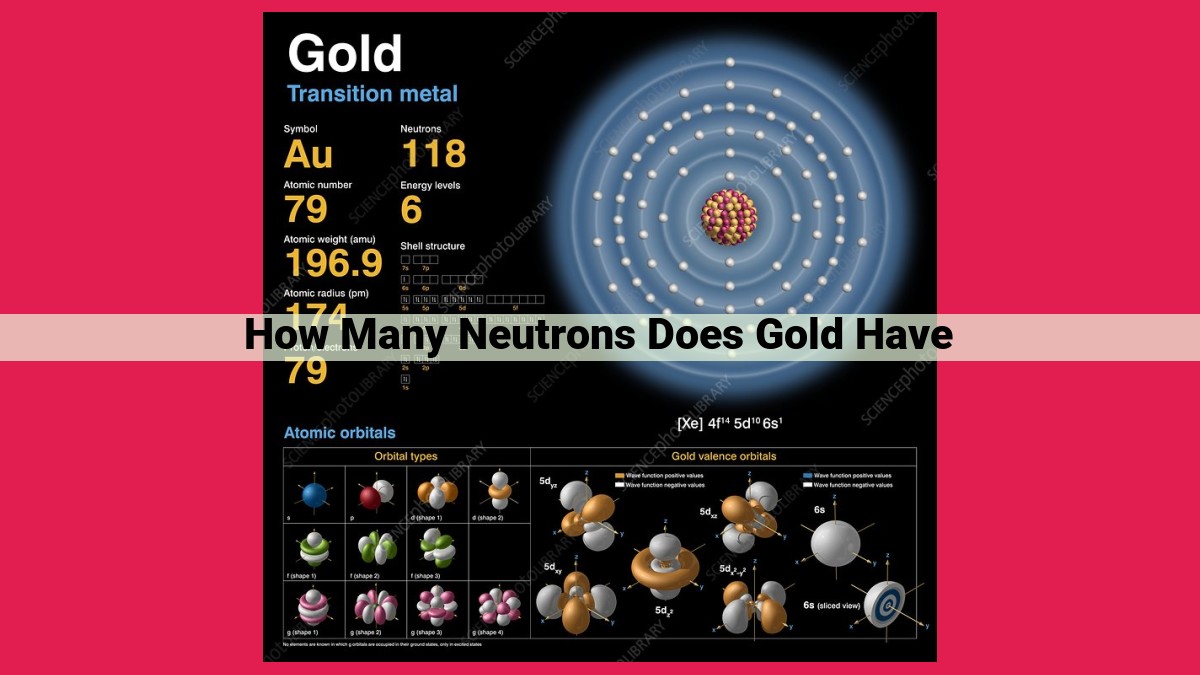
Neutrons, subatomic particles found in the nucleus, contribute to an atom’s mass. Gold, with an atomic number of 79, has a mass number of 197. By subtracting the atomic number from the mass number, we find that gold has 118 neutrons. This information is crucial for understanding gold’s atomic structure, as well as its applications in nuclear physics and material science.
- Introduce the topic: How many neutrons does gold have?
- Briefly explain the concept of neutrons and their role in atomic structure.
How Many Neutrons Does Gold Have: Unveiling the Secrets of the Noble Metal
Embark on a captivating journey into the realm of atomic structure as we delve into the enigmatic world of neutrons and their profound impact on the properties of gold. These elusive subatomic particles, often shrouded in mystery, play a pivotal role in shaping the very essence of this precious metal.
Neutrons, along with protons and electrons, are the fundamental building blocks of atoms. Residing within the heart of the atom, the nucleus, neutrons are the unsung heroes of atomic stability. Their presence ensures a harmonious balance within the nucleus, counteracting the repulsive forces between positively charged protons.
Gold’s allure stems not only from its radiant beauty but also from its unique atomic composition. Unveiling the number of neutrons in gold is akin to unlocking a secret vault, revealing the key to understanding its remarkable characteristics.
Neutrons in Atoms: Unveiling the Heart of Gold
Neutrons: The Silent Companions
At the heart of every atom, nestled alongside protons and electrons, lies a mysterious player: the neutron. These subatomic particles possess no electric charge, making them neutral in nature. They share the nucleus, which is the central dense core of the atom, with protons, which carry a positive charge.
The Location of Neutrons: A Nuclear Abode
Within the nucleus, neutrons reside in close proximity to protons, forming a nuclear bond through the strong nuclear force. This force is responsible for holding the atomic nucleus together against the repulsive electrostatic forces between positively charged protons. The neutron’s mass contributes to the overall mass of the nucleus and, subsequently, to the atom itself.
Neutron Number: A Defining Characteristic
The neutron number of an atom represents the number of neutrons present in its nucleus. This number, along with the atomic number (number of protons), determines the identity of an element and its unique properties.
Atomic Number and Protons: The Foundation of Element Identification
In the heart of every atom lies a microscopic universe, where the fundamental building blocks of matter dance in harmony. Among these, the protons hold a critical role, defining the very identity of each element.
The atomic number, represented by the symbol Z, is a unique identifier that tells us the number of protons nestled within an atom’s nucleus. It’s like a molecular fingerprint, distinguishing one element from another.
The protons are tiny particles with a positive electrical charge. They balance the electrons, negatively charged particles that orbit the nucleus. This delicate dance between positive and negative charges keeps the atom electrically neutral.
The atomic number not only identifies an element but also determines its chemical behavior. Elements with the same atomic number have similar chemical properties, forming the basis of the periodic table. This table organizes elements by their atomic number, revealing patterns and relationships that guide chemical reactions and shape the world around us.
Mass Number and Its Calculation
In the realm of atomic structure, understanding the makeup of an atom is crucial. Mass number plays a pivotal role in this endeavor, providing insights into an atom’s composition.
Defining Mass Number
The mass number of an atom represents the total number of protons and neutrons found within its nucleus. It is a crucial metric in characterizing the atom’s mass and distinguishing it from other isotopes of the same element.
Calculating Mass Number
Determining the mass number involves a straightforward calculation: adding the number of protons and neutrons present in the nucleus.
Mass number = Number of protons + Number of neutrons
This formula highlights the fact that protons and neutrons, despite their differences in electrical charge, contribute equally to the mass of the atom.
For instance, the mass number of an atom with 6 protons and 8 neutrons would be 14 (6 + 8 = 14). This information is essential for understanding the atomic structure and the element’s properties.
Unraveling the Neutron Enigma: How Many Does Gold Possess?
Atomic Number: Unlocking Gold’s Identity
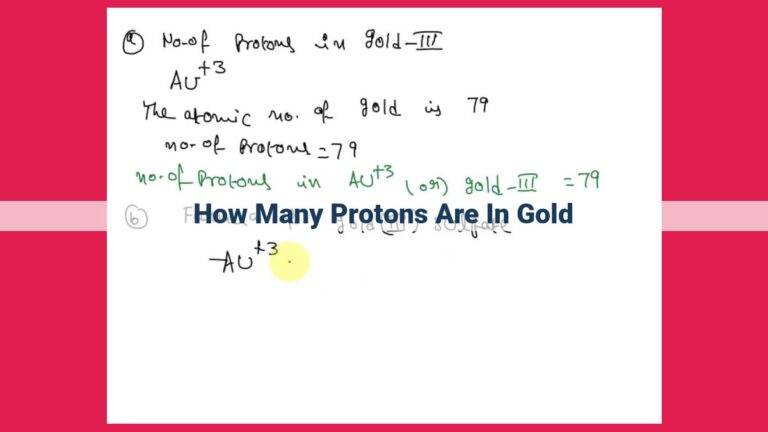
Like a meticulous fingerprint, each element boasts a unique atomic number, a numerical code that distinguishes it from its elemental peers. In gold’s case, this atomic fingerprint reads 79. This number represents the number of protons residing within its nucleus, the atom’s bustling heart.
Mass Number: A Measure of Atomic Heft
Mass number, a slightly bulkier cousin of the atomic number, embodies the total number of protons and neutrons snuggled within the nucleus. 197 serves as gold’s mass number, a reflection of its atomic heft.
Neutrons: The Unsung Nuclear Pillars
Neutrons, the silent partners of protons, share their nuclear abode. Unlike protons, which carry a positive charge, neutrons remain neutral, hence their name. Their presence lends stability to the nucleus, acting as a buffer between the positively charged protons.
Unveiling the Neutron Count: A Mathematical Adventure
Armed with gold’s atomic number of 79 and mass number of 197, we embark on a mathematical journey to unravel its neutron population. We simply subtract the atomic number from the mass number. Like peeling back layers of an onion, we find 118 neutrons dancing within gold’s nucleus.
Applications and Significance: Transcending Scientific Boundaries
Understanding the neutron count of gold extends beyond mere atomic curiosity. It finds practical applications in various scientific disciplines:
- Nuclear Physics: Neutrons play a pivotal role in nuclear reactions, including those harnessed for energy production and medical advancements.
- Material Science: The neutron composition influences a material’s physical and chemical properties, guiding its use in diverse industries.
Unveiling the neutron count of gold is akin to unlocking a treasure trove of atomic knowledge. It not only satisfies our scientific curiosity but also empowers us to harness gold’s unique properties for advancements that shape our world.
Unveiling the Secrets: How Many Neutrons Does Gold Hold?
Applications and Significance
Understanding the neutron composition of elements, like gold, extends beyond mere scientific curiosity. It holds practical implications and contributes significantly to scientific advancements.
In the realm of nuclear physics, the number of neutrons in gold plays a crucial role. The atomic nucleus, a miniature universe within the atom, harbors both protons and neutrons. The balance between these subatomic particles determines the stability of the nucleus. Gold, with its 79 protons and a varying number of neutrons, forms several isotopes. These isotopes have distinct properties due to their different neutron counts. This knowledge is essential for understanding nuclear reactions and the behavior of radioactive isotopes.
Beyond nuclear physics, the neutron composition of gold finds applications in material science. Gold’s unique properties, including its high density and electrical conductivity, make it a valuable material for various industries. By precisely controlling the number of neutrons in gold, scientists can tailor its properties for specific applications. For instance, in the biomedical field, gold nanoparticles with specific neutron compositions are used in drug delivery systems and diagnostic imaging.
Furthermore, unraveling the neutron composition of gold opens avenues for scientific research and technological advancements. The study of neutron behavior in gold and other elements provides insights into the fundamental forces that govern the universe. This knowledge has led to breakthroughs in particle physics and cosmology. Additionally, understanding neutron composition aids in the development of advanced materials with enhanced properties, paving the way for innovative technologies in fields such as electronics, energy, and medicine.