Understanding The Neutron Number: Unraveling Atomic Structure And Properties With Manganese-55
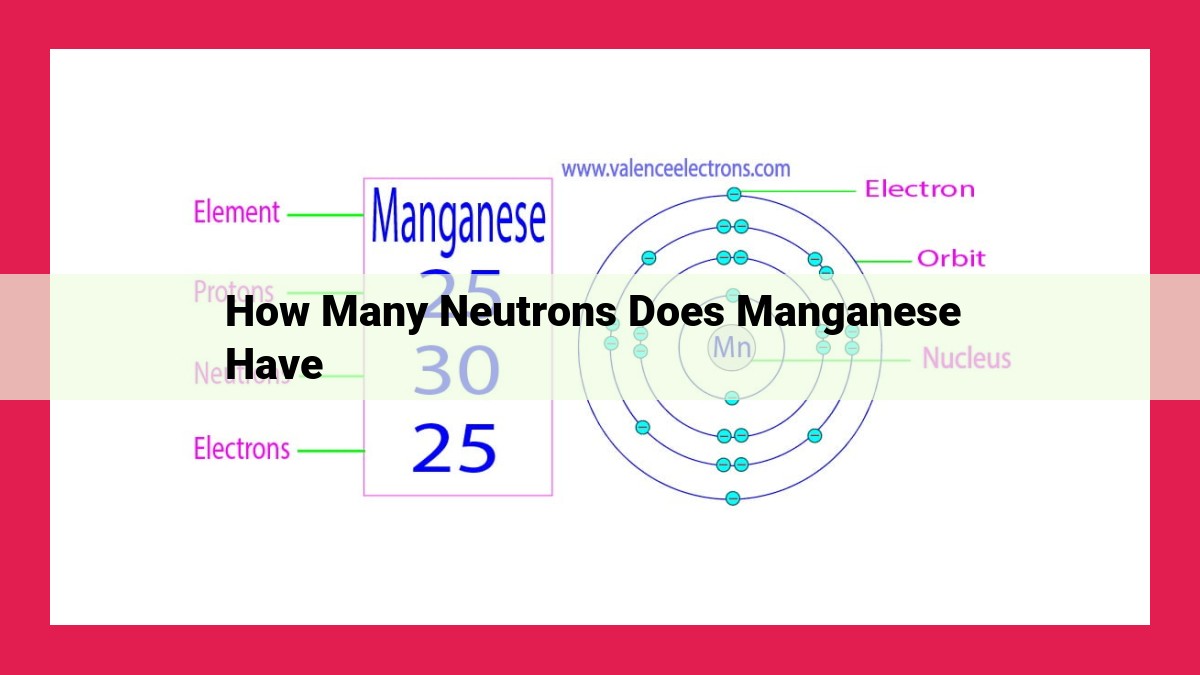
Manganese-55, a common isotope, has 30 neutrons. This is calculated by subtracting the atomic number (25, representing the number of protons) from the mass number (55, indicating the total number of protons and neutrons). The neutron number, which represents the number of neutrons in an atom, is crucial for understanding atomic structure and properties.
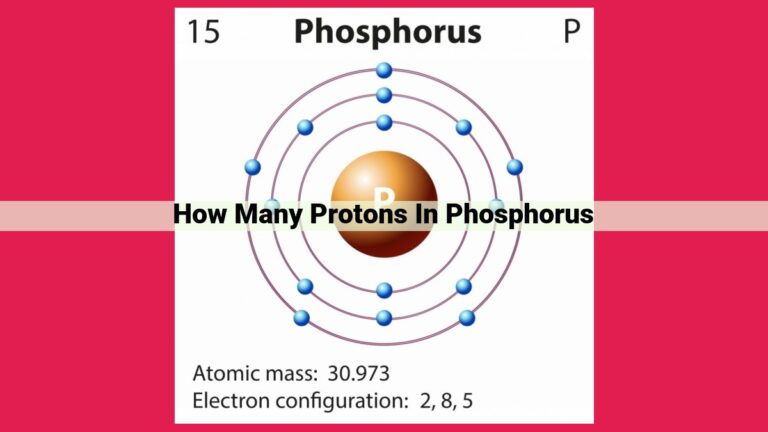
Atomic Number of Manganese
- Definition of atomic number
- Manganese’s atomic number (25)
Unveiling the Secrets of Manganese: Atomic Number and Structure
In the realm of chemistry, understanding the fundamental building blocks of elements is crucial. Let’s explore one such element, manganese, and delve into its atomic number.
The Atomic Number of Manganese: A Journey to Its Core
Every element is defined by its unique atomic number, which represents the number of positively charged protons in its nucleus. Manganese’s atomic number is 25, indicating that each atom of manganese contains 25 protons. This number is a constant that distinguishes manganese from all other elements.
Atomic Structure: Beyond Protons and Neutrons
The atomic number is intertwined with the concept of mass number, which denotes the total number of protons and neutrons combined. For the isotope manganese-55, the mass number is 55. This means that each atom of manganese-55 contains 25 protons and 30 neutrons.
Neutrons: The Silent Majority
The number of neutrons in an atom is known as its neutron number. This number can be calculated using the formula:
Neutron number = Mass number – Atomic number
For manganese-55, this calculation reveals 30 neutrons. These neutrons contribute to the overall mass of the atom but do not carry an electrical charge.
The Significance of Atomic Structure
Understanding the atomic structure of elements, including their atomic numbers and neutron numbers, is essential for comprehending their chemical properties and behavior. This knowledge plays a vital role in various fields, from quantum mechanics to nuclear physics.
In the case of manganese, its specific atomic and neutron numbers give it unique properties that make it indispensable in industries such as steel production, battery manufacturing, and water purification. By grasping the fundamentals of atomic structure, we unlock the door to understanding the intricate workings of the chemical world.
Mass Number of Manganese-55: Unveiling the Secrets of Atomic Structure
In the intricate realm of atomic chemistry, understanding the characteristics of elements is paramount. Among these essential properties, mass number holds significant importance. In this exploration, we will delve into the mass number of Manganese-55, its definition, significance, and how to determine it using a simple formula.
Unveiling the Essence of Mass Number
At its core, mass number represents the total number of protons and neutrons found within an atomic nucleus. Imagine an atom as a microscopic universe, where the nucleus, like a tiny, dense city, houses protons and neutrons, the fundamental building blocks of matter. Protons, carrying a positive charge, reside at the heart of the nucleus, while neutrons, their uncharged counterparts, orbit around them. Together, protons and neutrons determine the mass number of an atom.
Manganese-55: A Case Study in Atomic Structure
Manganese-55 is a specific isotope of the element manganese, denoted by the symbol ⁵⁵Mn. Like all isotopes, ⁵⁵Mn shares the same atomic number (25) as other manganese atoms, indicating the identical number of protons (25) in each nucleus. However, ⁵⁵Mn stands out due to its unique mass number of 55.
Calculating the Number of Neutrons: A Mathematical Dive
To determine the number of neutrons in ⁵⁵Mn, we employ a straightforward formula:
Neutrons = Mass number – Atomic number
Plugging in the values for ⁵⁵Mn, we get:
Neutrons = 55 – 25 = 30
This calculation reveals that ⁵⁵Mn contains 30 neutrons, which, combined with its 25 protons, contribute to its mass number of 55.
Comprehending atomic structure, including mass number, deepens our understanding of the elemental building blocks of our universe. ⁵⁵Mn, with its mass number of 55 and 30 neutrons, serves as a tangible example. Unraveling the mysteries of atomic structure empowers us to appreciate the intricate symphony of matter and to unlock its potential for advancing science and technology.
Unveiling the Secrets of Manganese-55: A Journey into Atomic Structure
Welcome to our atomic adventure, where we’ll delve into the fascinating world of manganese-55 and its enigmatic neutron count. But first, let’s lay the groundwork with a crash course in atomic terminology.
Atomic Number: A Unique Identity
Every element is defined by its atomic number, a unique number that represents the number of protons in its nucleus. Protons carry a positive charge, making them the balancing force to the negatively charged electrons that orbit the nucleus. Manganese proudly boasts an atomic number of 25, meaning it has 25 protons within its atomic core.
Mass Number: The Whole Enchilada
The mass number of an atom, on the other hand, encompasses not only the protons but also the neutrons, which reside in the nucleus alongside the protons. Unlike protons, neutrons have no electrical charge, hence their neutral status.
Calculating the Neutron Count: A Simple Formula
Now, for the moment you’ve been waiting for: calculating the number of neutrons in manganese-55. Here’s where the magic formula comes into play:
Neutrons = Mass number – Atomic number
Since we know manganese-55’s mass number is 55 and its atomic number is 25, we can do the math:
Neutrons = 55 – 25
Voila! Manganese-55 has 30 neutrons.
Neutron Number: A Pivotal Perspective
The neutron number, sometimes referred to as the neutron count, is simply the number of neutrons in an atom’s nucleus. In the case of manganese-55, its neutron number is 30, which provides a deeper understanding of its atomic composition and properties.
Our exploration has illuminated the atomic structure of manganese-55, revealing its atomic number of 25, mass number of 55, and neutron number of 30. Comprehending these fundamental concepts not only enhances our knowledge of atomic science but also underscores the significance of understanding the interplay between protons, neutrons, and electrons in determining an element’s characteristics.
So, there you have it – the intriguing tale of manganese-55’s neutron count, a testament to the intricacies of the atomic universe.
Neutron Number
- Definition of neutron number
- Manganese-55’s neutron number (30)
Neutron Number: Unveiling the Secrets of Manganese-55
In the fascinating realm of atomic structure, the neutron number plays a pivotal role in defining the composition of an element. This numerical value represents the number of neutrons present within the nucleus of an atom, offering insights into its overall stability and behavior.
Defining Neutron Number
Simply put, the neutron number is the difference between an atomic nucleus’s mass number and its atomic number. The atomic number, denoted by Z, represents the number of protons within the nucleus, while the mass number, denoted by A, represents the total number of protons and neutrons. Thus, the neutron number (N) is expressed as:
N = A - Z
Manganese-55: A Case Study
Let’s delve into the case of manganese-55, a naturally occurring isotope of manganese. Manganese-55 possesses a mass number of 55, indicating the presence of a total of 55 protons and neutrons within its nucleus. Its atomic number is 25, revealing the presence of 25 protons.
Calculating Manganese-55’s Neutron Number
Utilizing the formula mentioned earlier, we can effortlessly calculate the neutron number of manganese-55:
N = 55 (Mass number) - 25 (Atomic number)
N = 30
Significance of Neutron Number
The neutron number is a crucial factor in determining the stability, decay pathways, and reactivity of an element. The presence of an equal number of protons and neutrons generally leads to a more stable nucleus, while significant imbalances can result in radioactive decay.
In the case of manganese-55, the presence of 30 neutrons contributes to its overall stability and characteristic properties. Understanding the concept of neutron number and its implications deepens our comprehension of the intricate world of atomic structure, paving the way for advancements in various fields, including nuclear physics, chemistry, and materials science.