Understanding Neutralization Reactions: A Guide To Salt, Water, And Heat Formation
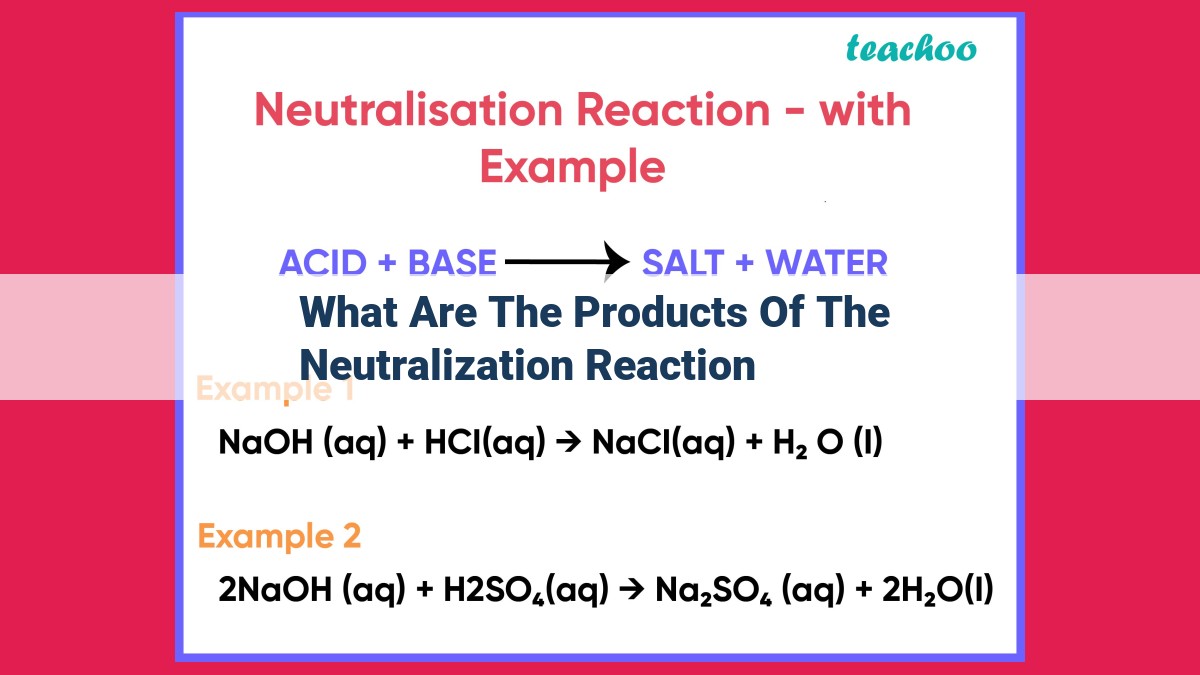
Neutralization reactions combine an acid and a base to yield a salt, water, and heat. The salt, formed from the base’s cation and the acid’s anion, is a neutral ionic compound that often exists in a crystalline state. Water, a polar molecule, is produced from the hydrogen ions of the acid and hydroxide ions of the base, forming covalent bonds. The reaction is exothermic, releasing heat energy as the ions combine to form stable compounds.
Neutralization Reactions: A Chemical Dance of Ions
In the realm of chemistry, a captivating tale unfolds when acids and bases encounter each other, leading to a dramatic transformation known as neutralization reactions. These reactions are like a dance, where the ions of the acid and base playfully collide and transform into substances with entirely different properties.
Neutralization reactions most commonly occur between acids, which donate hydrogen ions (H+), and bases, which accept hydrogen ions. As they combine, these oppositely charged ions neutralize each other, creating salt and water. Salt is a compound composed of positively charged metal ions and negatively charged nonmetal ions, while water is a deceptively simple molecule that plays a vital role in life processes.
Salt: The Salty Story of Neutralization Reactions
In the realm of chemistry, neutralization reactions play a pivotal role, resulting in the formation of salt. Salt, a ubiquitous culinary staple, holds a wealth of scientific intrigue woven into its crystalline structure.
The Birth of Salt
When acids and bases engage in a chemical dance, they undergo a transformation, giving rise to salt. Acids, characterized by their sour taste and ability to donate protons (H+), encounter bases, which are bitter in taste and accept protons.
The resulting union between acids and bases produces two distinct products: salt and water. Salt, composed of positive and negative ions, is an ionic compound, meaning its constituent ions are held together by electrostatic forces.
The Alchemy of Ion Exchange
The base ions within salt originate from the base that participated in the neutralization reaction, while the acid ions stem from the acid. For instance, in the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH), sodium ions (Na+) from NaOH combine with chloride ions (Cl-) from HCl to form sodium chloride (NaCl) – the common table salt.
This ion exchange is a crucial step in the formation of salt. The combination of oppositely charged ions creates a stable ionic compound.
The Significance of Salt
Beyond its culinary significance, salt plays a multifaceted role in various scientific and industrial applications. Its ability to conduct electricity makes it essential for batteries and electrolytic cells. In medicine, it serves as a disinfectant and electrolyte in intravenous solutions.
Furthermore, salt’s role in the preservation of food cannot be underestimated. It inhibits the growth of microorganisms by drawing out moisture from their cells, extending the shelf life of many edibles.
By understanding the formation, composition, and properties of salt, we gain a deeper appreciation for the intricate workings of chemical reactions. Neutralization reactions are not mere laboratory curiosities but lie at the heart of countless everyday phenomena, from the taste of our food to the preservation of our health.
Water: A Vital Product of Neutralization Reactions
In the realm of chemical reactions, neutralization stands out as a transformative process that produces two essential substances: salt and water. Water, in particular, plays a pivotal role in this reaction, not only as a product but also as a crucial element in shaping its properties.
Formation and Molecular Structure
Neutralization reactions occur when an acid and a base interact, leading to the formation of a salt and water. During this process, hydrogen ions (H+) from the acid combine with hydroxide ions (OH-) from the base, resulting in the formation of water molecules (H2O).
Water molecules are unique in their molecular structure. They consist of two hydrogen atoms covalently bonded to a central oxygen atom. The hydrogen atoms form an angle of 104.5 degrees, resulting in a bent or “V” shape. This molecular geometry gives water its polar nature, with the oxygen atom being slightly negative and the hydrogen atoms being slightly positive.
Chemical Interactions
The chemical interactions between hydrogen and oxygen in water are governed by the concept of electronegativity. Electronegativity refers to an atom’s ability to attract electrons towards itself. Oxygen is more electronegative than hydrogen, meaning it has a stronger pull on the shared electrons in the covalent bonds.
As a result, the electron density is shifted towards the oxygen atom, creating a slight negative dipole on the oxygen atom and a slight positive dipole on each hydrogen atom. This polarity is responsible for water’s unique properties, such as its high surface tension and its ability to dissolve many substances.
Ionization and PolarITY
The polarity of water plays a crucial role in its ability to ionize. Ionization refers to the process of atoms or molecules gaining or losing electrons, resulting in the formation of ions. In water, a small fraction of molecules ionize, forming hydrogen ions (H+) and hydroxide ions (OH-).
The presence of these ions is essential for understanding neutralization reactions. When an acid and a base are combined, the hydrogen ions from the acid react with the hydroxide ions from the base to form water. This reaction neutralizes the charges of the ions, resulting in the formation of a salt and water.
Water is not merely a byproduct of neutralization reactions; it is a vital substance that plays a crucial role in shaping the properties of the reaction. Its molecular structure, chemical interactions, polarity, and ability to ionize all contribute to its significance in understanding and utilizing neutralization reactions in various applications.
Heat: Energy Change in Neutralization Reactions
Neutralization reactions are exothermic processes, meaning they release heat energy as products are formed. Imagine yourself dissolving a strong acid like hydrochloric acid (HCl) in water, creating hydronium ions (H3O+) and chloride ions (Cl-). Now, let’s introduce a strong base like sodium hydroxide (NaOH), which dissociates into sodium ions (Na+) and hydroxide ions (OH-).
As the hydronium and hydroxide ions encounter each other, a vigorous reaction ensues. The oppositely charged ions are drawn together, neutralizing their charges and forming water molecules (H2O). Simultaneously, the excess energy released during this neutralization is dissipated as heat. You may even feel a noticeable temperature increase if the reaction is conducted in a test tube.
This transfer of energy is significant. The breaking of the ionic bonds in the acid and base releases energy, while the formation of new covalent bonds in water absorbs energy. The net result is an exothermic reaction, with the excess energy released as heat. This heat energy can be used for various practical applications, such as generating steam for power plants or providing warmth in chemical hand warmers.
Neutralization Reaction: Unraveling the Process
Neutralization reactions, like magic tricks of chemistry, transform two seemingly incompatible substances – an acid and a base – into a harmonious duo of salt and water. Let’s embark on a step-by-step journey to unravel this chemical wonderland.
Step 1: The Clash of Titans
When an acid (characterized by its tartness and ability to donate hydrogen ions or H+) encounters a base (known for its bitter taste and willingness to accept H+ ions), a chemical dance ensues. The H+ ions from the acid eagerly rush towards the base, like a knight charging into battle.
Step 2: Ionic Embrace
As the H+ ions bond with the OH- ions (hydroxide ions) from the base, they create a beautiful bond known as water (H2O). Meanwhile, the remaining positive ions from the acid and the negative ions from the base form a new substance called salt.
Step 3: The Energetic Aftermath
This chemical union is not without its fiery side effects. Neutralization reactions release heat, making them exothermic. Energy is transferred from the reactants (acid and base) to the products (salt and water). This energy release can cause a noticeable temperature increase in the reaction mixture.
Step 4: The Product Showcase
The salt, a neutral substance, is a product of this chemical alchemy. It possesses unique properties and characteristics that depend on the specific acid and base involved. The water, on the other hand, is a universal solvent known for its life-sustaining properties.
Step 5: The Balancing Act
To achieve a perfect balance in a neutralization reaction, the amount of acid and base used must be carefully calculated. If one substance is present in excess, it will remain unreacted and alter the properties of the final solution.
Concepts in Neutralization Reactions
To delve into the intricacies of neutralization reactions, we must first establish a firm understanding of the underlying concepts.
-
Acids: These are substances that contain hydrogen ions (H+) and can donate them to other substances. Acids typically have a sour taste and can react with metals to produce hydrogen gas.
-
Bases: Substances that accept hydrogen ions (H+) are known as bases. They usually have a bitter taste and feel slippery when touched. Bases can neutralize acids, forming salt and water.
-
Ionization: The process by which a substance loses or gains electrons, forming ions, is called ionization. In neutralization reactions, acids and bases ionize in water, releasing their respective ions.
-
Polarity: The uneven distribution of electrons within a molecule results in polarity. Polar molecules have a positive end and a negative end. These polarities play a crucial role in the interactions between acids, bases, and water molecules.
These concepts are inextricably linked to the understanding of neutralization reactions. Acids and bases, upon reacting, neutralize each other, forming salt and water. This reaction is driven by the exchange of ions, facilitated by the ionization process. The polarity of water molecules allows them to dissolve ionic compounds, further enhancing the reaction’s progress. By understanding these concepts, we can better grasp the nature and significance of neutralization reactions.