Neutralization Of Hydrochloric Acid: Titration, Equivalence Point, And Indicator Selection
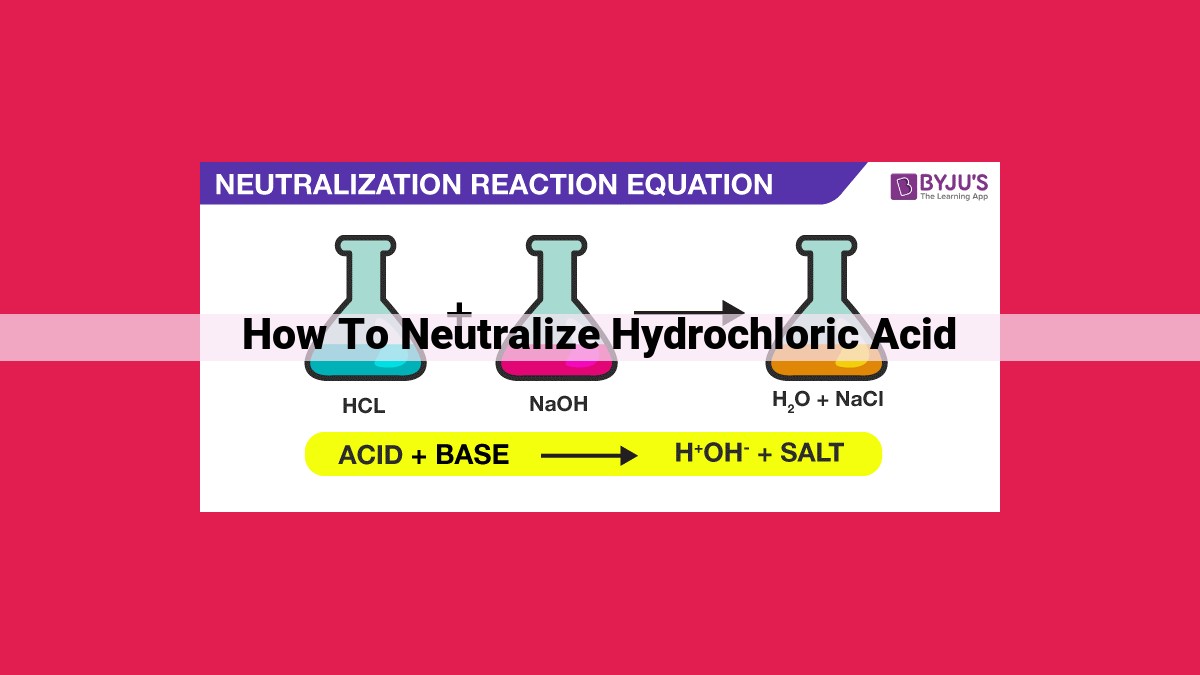
Neutralizing hydrochloric acid (HCl) involves a chemical reaction with a base to form a salt and water. The equivalence point, determined through titration, indicates the complete reaction of acid and base. Acid-base indicators, such as phenolphthalein, facilitate visual detection of the endpoint, where the solution turns a specific color. Strong bases like sodium hydroxide (NaOH) neutralize HCl more effectively than weak bases like sodium carbonate (Na2CO3) due to their higher dissociation and hence, greater availability of hydroxide ions.
Understanding Neutralization Reactions: A Chemical Balancing Act
In the world of chemistry, acids and bases are like feuding neighbors who, when brought together, form a harmonious union. This magical process, known as neutralization, is a chemical reaction that occurs between an acid and a base to form salt and water.
Hydrochloric acid (HCl), a common acid, is a prime example of an acid that undergoes neutralization. When HCl meets a base, the two dance a delicate dance, exchanging ions that create a salt and water. The salt, a neutral compound, is essentially the tamed version of the acid and base. Water, a life-giving molecule, is the byproduct of this neutralized encounter.
Acids, bases, and water each bring their own unique properties to the neutralization process. Acids, like HCl, are characterized by their sour taste, ability to react with metals, and turn litmus paper red. Bases, on the other hand, are bitter to the taste, feel soapy to the touch, and turn litmus paper blue. Water, the universal solvent, is present in all neutralization reactions as a neutral medium that helps facilitate the exchange of ions.
Determining the Equivalence Point in Neutralization Reactions
In a captivating tale of chemical harmony, neutralization reactions unite acids and bases, yielding the serene balance of salts and water. Understanding the equivalence point is crucial in this dance, for it marks the moment of perfect equilibrium.
The equivalence point, like a celestial meeting spot, occurs when the moles of acid and base are equal. Imagine a gentle dance between hydrochloric acid (HCl), a strong acid, and a gentle base. As they waltz, each molecule of HCl gracefully pairs with an OH- ion from the base, forming water.
Calculating the equivalence point is a delicate balancing act. It requires the deft art of stoichiometry, where the secrets of moles and chemical equations are revealed. By understanding the mole ratio of acid to base, we can predict the exact moment when their union reaches its zenith.
For instance, consider the reaction between HCl and sodium hydroxide (NaOH):
HCl + NaOH → NaCl + H2O
The mole ratio tells us that 1 mole of HCl reacts with 1 mole of NaOH. Thus, to determine the equivalence point, we simply equate the moles of HCl and NaOH:
Moles of HCl = Moles of NaOH
By solving this equation, we unveil the precise volume of base needed to neutralize a given volume of acid. The equivalence point, where harmony reigns, has been found.
In conclusion, determining the equivalence point in neutralization reactions is a dance of precision, a step towards understanding the transformative power of chemical interactions. By mastering the art of stoichiometry, we unravel the secrets of balance and equilibrium, revealing the hidden beauty within the chemical realm.
Titration: Unveiling the Mysteries of Acid-Base Reactions
In the realm of chemistry, neutralization reactions play a crucial role in understanding the behavior of acids and bases. Titration emerges as a valuable tool that allows us to unravel the secrets of these reactions and determine the concentration of acids with precision.
What is Titration?
Titration is a laboratory technique that involves the controlled addition of a known reagent, called the titrant, to a solution of unknown concentration, known as the analyte. The goal is to find the equivalence point, where the moles of acid and base are equal, indicating complete neutralization.
Measuring the Concentration of HCl with Titration
Hydrochloric acid (HCl) is a common acid used in titration experiments. To determine its concentration, we use a strong base as the titrant. As the base is gradually added to the HCl solution, the pH of the mixture changes.
The Role of pH in Determining the Equivalence Point
The pH value is a measure of the acidity or basicity of a solution. At the equivalence point in a neutralization reaction, the pH will be neutral, indicating that the solution contains equal amounts of acid and base.
Using Indicators to Detect the Endpoint
Determining the exact equivalence point can be challenging, so we use acid-base indicators, which change color at a specific pH range. One commonly used indicator for neutralizing HCl is phenolphthalein, which turns colorless in an acidic solution and pink in a basic solution. As we approach the equivalence point, the indicator will exhibit a gradual color change, providing a visual cue.
By carefully observing the color change of the indicator, we can identify the endpoint of the titration, which is very close to the equivalence point. The endpoint is the point at which the indicator has completely changed color, indicating that the reaction is complete.
Therefore, titration becomes a practical approach to measure the concentration of HCl by accurately determining the equivalence point, where the acid and base are completely neutralized.
Acid-Base Indicators: Visual Cues in Neutralization Reactions
In the realm of chemistry, neutralization reactions play a crucial role, involving the reaction between acids and bases to form neutral salts and water. Acid-base indicators, like trusty detectives, assist us in determining the pivotal equivalence point—the moment when the moles of acid and base are in perfect balance. These indicators are the eyes of the chemist, signaling the endpoint of the reaction through noticeable color changes.
Acid-base indicators are weak acids or bases themselves. They exist in two distinct forms: the acidic form and the basic form. Each form has its characteristic color. The pH-dependent nature of these indicators allows them to undergo color changes at specific pH values.
Selecting the appropriate indicator for a particular neutralization reaction is crucial. The indicator should change color near the equivalence point of the reaction. For instance, in the neutralization of hydrochloric acid (HCl), an acid with a relatively low pH, phenolphthalein is a widely used indicator. This is because phenolphthalein undergoes a colorless to pink transition at a pH of around 8.3, which is close to the equivalence point of HCl neutralization.
Acid-base indicators are not only fascinating chemical tools but also essential allies in the laboratory. They provide visual cues, enabling us to determine the endpoint of neutralization reactions accurately and efficiently.
Phenolphthalein: The Perfect Indicator for Neutralizing Hydrochloric Acid
In the realm of chemistry, neutralization reactions are like a delicate dance between acids and bases. When these reactants come together, they form salts and water, creating a harmonious balance. To witness this fascinating process, scientists use a special tool called an indicator, and one of the most trusted indicators for neutralizing hydrochloric acid (HCl) is phenolphthalein.
Phenolphthalein is a weak acid-base indicator that undergoes a dramatic color change as the pH of a solution shifts. In acidic solutions, it remains colorless and inconspicuous. However, as the pH increases towards neutrality, phenolphthalein springs to life, transforming into a vibrant pink hue.
This pH-dependent color change makes phenolphthalein an invaluable tool in titration, a technique used to determine the concentration of an unknown acid or base. When added to a solution of HCl, phenolphthalein acts as a silent observer, patiently waiting for the moment when the equivalence point is reached.
The equivalence point, a crucial landmark in neutralization, represents the moment when the moles of acid and base are exactly equal. At this precise instant, the solution becomes perfectly neutral, and phenolphthalein bursts into color, signaling that the reaction is complete.
The specificity of phenolphthalein for the neutralization of HCl makes it an ideal choice for this particular reaction. Its color change occurs at a pH of approximately 8.2, which coincides with the equivalence point of HCl with most strong bases.
In the laboratory, phenolphthalein plays a vital role in guiding scientists through the neutralization process. As the base is slowly added to the HCl solution, the colorless phenolphthalein remains passive. But as the equivalence point approaches, the solution begins to take on a faint pink tinge. With each drop of base, the pink color intensifies until, at the magic moment of equivalence, the solution transforms into a vibrant shade that signals the completion of the reaction.
Phenolphthalein, with its pH-dependent color change and specificity for HCl neutralization, serves as a reliable guide in the fascinating dance of neutralization reactions. It allows scientists to witness the harmonious union of acids and bases, and to determine the exact moment when the perfect balance is achieved.
Strong vs. Weak Bases in Neutralization: Unraveling the Differences
In the realm of chemistry, acids and bases play a crucial role in neutralization reactions, where they react to form salts and water. Among bases, we encounter a fascinating distinction between strong and weak bases, each with unique properties that influence their effectiveness in neutralization.
Strong Bases: The Powerhouses of Neutralization
Strong bases like sodium hydroxide (NaOH) possess a remarkable ability to ionize completely in water, releasing hydroxide ions (OH-) in abundance. Their strong nature stems from their complete dissociation, resulting in a high concentration of OH- ions.
Weak Bases: The Underdogs of Neutralization
Weak bases like sodium carbonate (Na2CO3), on the other hand, exhibit a more subtle ionization behavior. They only partially dissociate in water, releasing a lower concentration of OH- ions. This partial dissociation limits their ability to neutralize acids compared to their stronger counterparts.
Neutralization Capacity: A Measure of Effectiveness
The neutralization capacity of a base refers to its ability to react with and neutralize acids. Strong bases, armed with their high OH- ion concentration, boast a greater neutralization capacity. They can effectively neutralize a larger amount of acid before the equivalence point is reached.
Examples in Action
To illustrate this difference, let’s consider the neutralization of hydrochloric acid (HCl):
- When sodium hydroxide (NaOH), a strong base, is added to HCl, it neutralizes the acid rapidly and completely, forming sodium chloride (NaCl) and water.
- Sodium carbonate (Na2CO3), a weak base, neutralizes HCl gradually and requires a larger amount to reach the equivalence point. This is due to its lower OH- ion concentration.
Understanding the distinction between strong and weak bases is essential for comprehending neutralization reactions. Strong bases exhibit greater neutralization capacity due to their complete ionization, while weak bases have a limited capacity due to their partial dissociation. This knowledge aids in selecting the appropriate base for specific neutralization applications.