Understanding Negative Enthalpy Change: Exothermic Reactions Explained For Seo
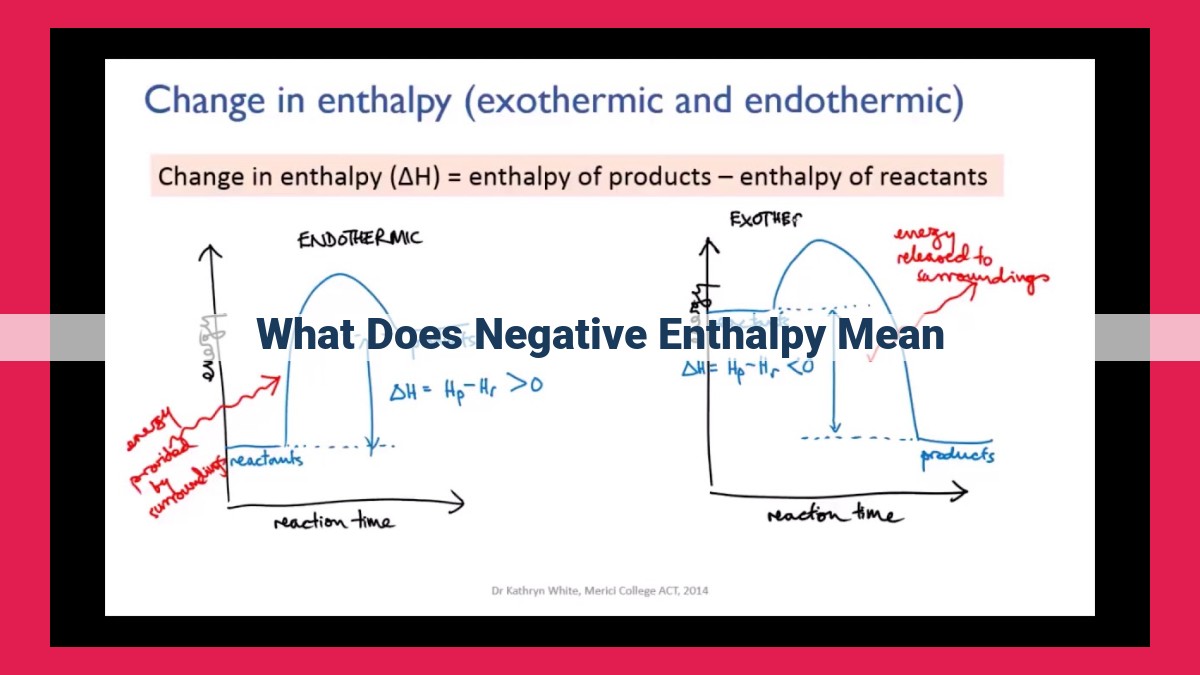
Negative enthalpy change (ΔH < 0) indicates an exothermic reaction, where the products have lower energy than the reactants. This results in the release of heat into the surroundings. Exothermic reactions are favored reactions that occur spontaneously. Negative enthalpy change is a key factor in determining the favorability and exothermic nature of chemical reactions.
Understanding Enthalpy and Its Impact on Chemical Reactions
Enthalpy: The Energy Behind Chemical Reactions
Enthalpy (H) is a critical thermodynamic property that measures the total thermal energy of a system, including its internal energy and the work it can perform. In chemical reactions, enthalpy change (ΔH) plays a crucial role in determining the reaction’s spontaneity and energy flow.
Negative Enthalpy Change: Exothermic Reactions
When a reaction releases heat into its surroundings, ΔH is negative. Such reactions are known as exothermic reactions. The negative ΔH indicates that the products have a lower enthalpy than the reactants. This energy difference is released as heat, causing the surroundings to become warmer.
Characteristics of Exothermic Reactions
Exothermic reactions exhibit distinct characteristics:
- Heat Release: The release of heat energy is a hallmark of exothermic reactions, increasing the temperature of their surroundings.
- Stability of Products: Exothermic reactions produce products with lower enthalpy, making them more stable than the reactants.
- Spontaneity: Exothermic reactions tend to occur spontaneously, driven by the decrease in overall enthalpy.
Favored Reactions: A Consequence of Negative Enthalpy Change
A negative ΔH is a sign of a favored reaction. Favored reactions are those that occur spontaneously, without the need for external energy input. The release of heat energy during an exothermic reaction creates a driving force that favors the formation of products.
Interplay of Enthalpy Change, Exothermic Reactions, and Favored Reactions
Enthalpy change, exothermic reactions, and favored reactions are intricately connected:
- ΔH determines the exothermic nature of a reaction.
- Exothermic reactions result in a negative ΔH.
- Negative ΔH indicates a favored reaction.
Understanding enthalpy change provides valuable insights into chemical reactions. It allows us to predict their spontaneity, energy flow, and favorability. Enthalpy change is a fundamental property that shapes the behavior of chemical systems and plays a vital role in energy transformations in chemistry and beyond.
Negative Enthalpy Change: Unraveling the Enigma of Exothermic Reactions
In the captivating world of chemistry, energy plays a pivotal role in dictating the course of reactions. Enthalpy change (ΔH) stands as a crucial indicator of the energy flow during chemical transformations. A negative enthalpy change (ΔH < 0) unveils a remarkable chapter in this tale, signaling the advent of exothermic reactions.
Exothermic reactions are akin to generous souls, readily releasing their warmth into the surroundings. As reactants embark on their transformative journey, bonds are broken and new ones are forged. This rearrangement releases energy in the form of heat, making the surroundings feel cozy and warm.
Think of a crackling fire on a cold winter’s night. The burning wood undergoes exothermic reactions, releasing heat that banishes the chill. Similarly, in the realm of chemistry, exothermic reactions bring forth a surge of energy, illuminating the path to spontaneous and favored reactions.
Spontaneous reactions, like a ball eagerly rolling downhill, proceed willingly without any external energy input. Favored reactions, guided by a negative enthalpy change, flow effortlessly in a downward energetic gradient. The liberation of heat during exothermic reactions fuels their inherent favorability.
Enthalpy change, exothermic reactions, and favored reactions intertwine like threads in an intricate tapestry. Understanding these concepts empowers us to unravel the mysteries of chemical transformations, predicting reaction outcomes and unraveling the energetic dance that unfolds within chemical processes.
Characteristics of Exothermic Reactions
In the realm of chemistry, reactions unfold as a captivating dance of energy exchange. Exothermic reactions stand out in this performance as the graceful partners that release heat energy into the surroundings. Their defining features paint a vivid picture of this energetic release, guiding us toward a deeper understanding of their behavior.
-
A Warm Embrace: Exothermic reactions are the givers of the chemical world. As they proceed, they willingly shed their energy like a blazing hearth, warming the environment around them. This exothermic process paints a vibrant contrast to its counterpart, the endothermic reaction, which greedily absorbs heat from its surroundings.
-
Unleashing Energy: The enthalpy change (ΔH) of an exothermic reaction dips below zero, symbolizing the liberation of heat energy. Imagine a ball rolling down a slope, losing potential energy as it descends. Similarly, in an exothermic reaction, the reactants possess higher energy than the products, and this energy difference is released as heat.
-
A Cascade of Light and Heat: The energy released during an exothermic reaction can manifest in various forms. Some reactions, like the combustion of fuels, produce blazing flames, a mesmerizing display of light and heat. Others, such as the neutralization of acids and bases, generate a gentle warmth, a testament to the energy released in the formation of new bonds.
-
Spontaneous and Favored: The negative enthalpy change of exothermic reactions favors their spontaneity. These reactions proceed eagerly, without the need for an external energy input. Their exothermic nature drives them forward, making them the preferred path for chemical transformations.
Understanding the characteristics of exothermic reactions is a key to unlocking the mysteries of chemical processes. By delving into their energy-releasing behavior, we gain insights into the dynamic interplay of energy and matter, and the remarkable transformations that shape our world.
**Favored Reactions: A Consequence of Negative Enthalpy Change**
In the realm of chemistry, spontaneous reactions hold a special allure, occurring effortlessly and seemingly without external influence. These reactions are known as favored reactions. But what lies beneath this spontaneous nature? The answer lies in the enigmatic concept of enthalpy change.
Enthalpy change (ΔH), represented as a positive or negative value, measures the energy flow during a chemical reaction. A negative ΔH signifies an exothermic reaction, where heat is released into the surroundings. Conversely, a positive ΔH indicates an endothermic reaction, where heat is absorbed from the surroundings.
The spontaneous nature of favored reactions arises from their negative ΔH. In exothermic reactions, the release of heat energy into the surroundings stabilizes the reaction products, making them more favorable. The decrease in enthalpy drives the reaction forward, leading to the spontaneous formation of products.
The connection between favored reactions and negative ΔH becomes even more evident when we examine the activation energy required for a reaction to occur. Activation energy is the minimum amount of energy that must be overcome to initiate a reaction. In favored reactions, the negative ΔH reduces the activation energy barrier, making it easier for the reaction to proceed spontaneously.
Thus, favored reactions are characterized by a negative ΔH and exothermic nature. Their spontaneity stems from the energy released during the reaction, which stabilizes the products and reduces the activation energy required for the reaction to occur. Understanding the interplay between enthalpy change and favored reactions provides a deeper insight into the dynamics of chemical processes and their applications in various fields.
Interconnections between Enthalpy Change, Exothermic Reactions, and Favored Reactions
- Highlight the interrelationship between these concepts
- Explain how enthalpy change determines the favorability and exothermic nature of reactions
Interconnections between Enthalpy Change, Exothermic Reactions, and Favored Reactions
In the realm of chemistry, enthalpy change (ΔH) plays a pivotal role in unraveling the mysteries of chemical reactions. It serves as a window into the energy dynamics of these processes, illuminating their spontaneity and energetic profiles.
A negative enthalpy change (ΔH < 0) is a beacon of exothermic reactions, signaling their tendency to release heat into the surroundings. These reactions are characterized by a spontaneous flow of energy, as the reactants’ stored chemical energy is converted into heat. This liberated heat can manifest as a surge in temperature, causing the reaction mixture to warm.
The interrelationship between enthalpy change, exothermic reactions, and favored reactions forms an intricate tapestry. Favored reactions are reactions that occur spontaneously, meaning they do not require an external energetic push. This spontaneity is intimately tied to negative enthalpy change.
The reason for this connection lies in the concept of Gibbs free energy. Gibbs free energy, a fundamental thermodynamic quantity, dictates the spontaneity of reactions. A negative Gibbs free energy change signifies a favored reaction. Significantly, enthalpy change is an integral component of Gibbs free energy, along with entropy change.
Thus, a negative enthalpy change (ΔH < 0), indicative of an exothermic reaction, drives a negative Gibbs free energy change, which in turn promotes favorability. In other words, reactions that release heat are more likely to occur spontaneously.
These concepts converge to form a powerful tool for predicting reaction outcomes and understanding the energetic flow in chemical processes. Enthalpy change, exothermic reactions, and favored reactions are interconnected, providing a framework for deciphering the chemistry that shapes our world.