Discover The Mystery Of Aluminum Isotopes: Unveiling The Neutron-Proton Dance
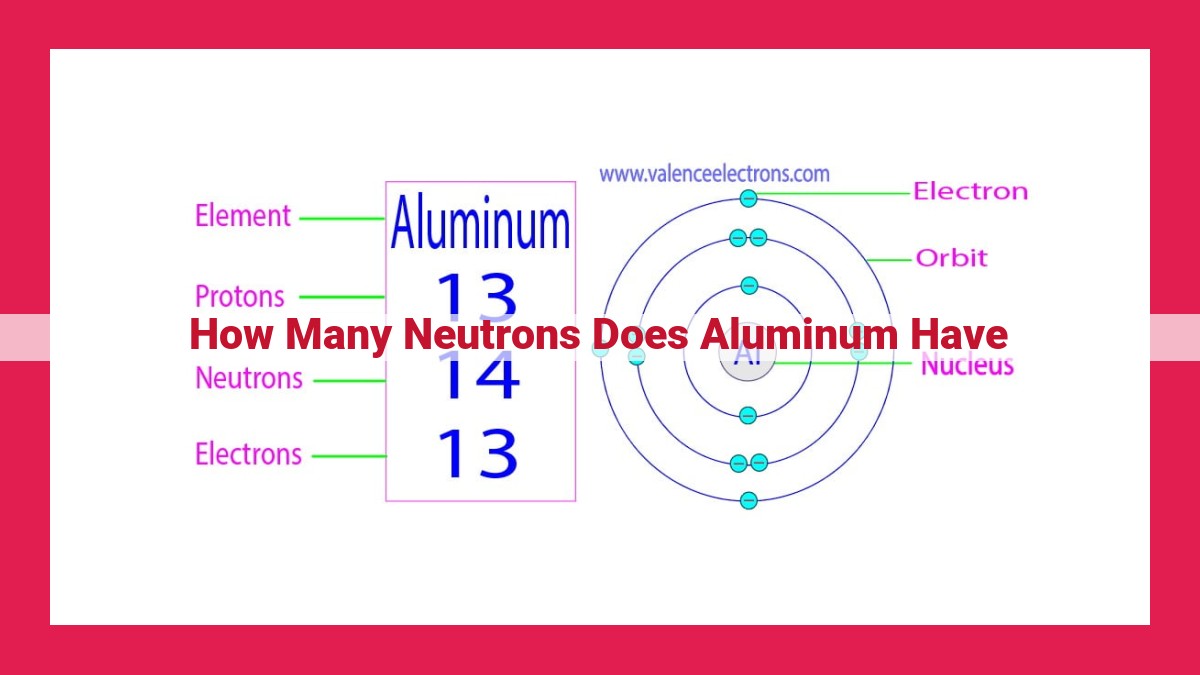
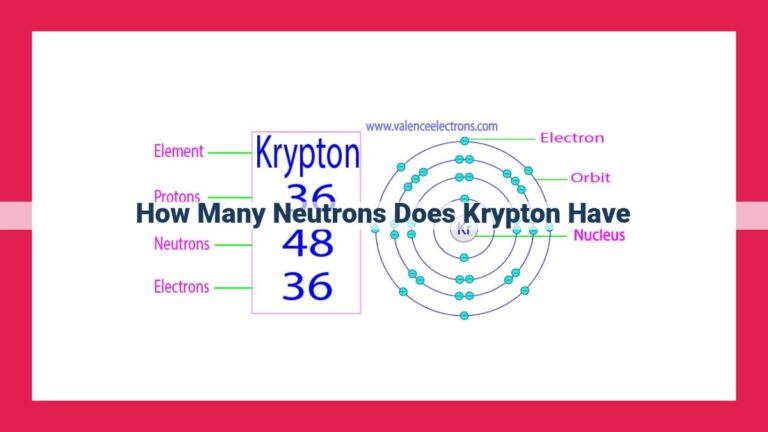
Aluminum’s number of neutrons depends on its isotope. The most common isotope, aluminum-27, has 13 neutrons, resulting in a mass number of 27. With an atomic number of 13, aluminum has 13 protons. Aluminum has various isotopes, including aluminum-26, aluminum-27, and aluminum-28, which differ in neutron count and slightly in atomic mass. In aluminum-27, the neutron-to-proton ratio is approximately 1:1.
Number of Neutrons in Aluminum
- Explain that the number of neutrons in aluminum varies depending on its isotope.
- Highlight that the most common isotope, aluminum-27, has 13 neutrons.
The Fascinating World of Aluminum: Unraveling Its Neutron Mystery
Aluminum, a lightweight yet durable metal, holds a captivating story within its atomic structure. One intriguing aspect of this element is the varying number of neutrons it possesses.
Neutron Variations: A Tale of Isotopes
In the realm of aluminum, the number of neutrons is not a fixed entity. This is because aluminum exists in various forms known as isotopes. Each isotope differs in the number of neutrons it contains, while sharing the same number of protons. The most prevalent aluminum isotope is aluminum-27, boasting 13 neutrons and 13 protons, giving it a total mass number of 27.
Understanding Mass Number and Atomic Number
The mass number of an atom represents the total number of protons and neutrons within its nucleus. In the case of aluminum-27, its mass number is 27. On the other hand, the atomic number of an element defines the number of protons in its nucleus. For aluminum, its atomic number is 13, which is a crucial factor in determining its position on the periodic table.
Understanding the Mass Number of Aluminum
Every element in the universe consists of tiny building blocks called atoms. At the heart of these atoms lies the nucleus, a dense core made up of protons and neutrons. The mass number of an element tells us the total number of protons and neutrons packed into its nucleus.
Aluminum, a lightweight and versatile metal, has a mass number that varies depending on its isotope. Isotopes are different forms of the same element, each with a unique combination of protons and neutrons. The most common isotope of aluminum, aluminum-27, has a mass number of 27. This means that each atom of aluminum-27 contains 13 protons (its atomic number) and 14 neutrons.
To calculate the mass number, we simply add up the number of protons and neutrons in the nucleus. For aluminum-27, this calculation looks like:
Mass number = Number of protons + Number of neutrons
Mass number = 13 + 14
Mass number = 27
The mass number plays a crucial role in understanding an element’s properties. It helps identify different isotopes, determine atomic masses, and predict nuclear reactions. By understanding the mass number of aluminum and its isotopes, scientists and engineers can tailor the use of this versatile metal for a wide range of applications.
The Atomic Number of Aluminum: Unraveling the Essence of an Element
In the captivating realm of chemistry, the atomic number stands as a defining characteristic of every element, a numerical fingerprint that unveils its unique identity. For the ubiquitous metal aluminum, this number is 13, a testament to the 13 protons that reside within its nucleus.
The atomic number, etched into the very essence of an element, serves as its passport to the periodic table. It determines the element’s place in the periodic sequence, a roadmap that arranges elements based on their shared properties. Aluminum finds its home in Group 13, alongside its fellow trivalent companions.
Within the bustling nucleus of an aluminum atom, protons and neutrons dance in a delicate equilibrium. Protons, bearing a positive charge, and neutrons, their neutral counterparts, form the core of the atom. It is the balance between these two particles that shapes the neutron-to-proton ratio, a crucial indicator of an element’s stability. In the case of aluminum, this ratio is approximately 1:1, indicating an equal number of neutrons and protons in its stable isotope, aluminum-27.
This delicate balance has profound implications for the physical and chemical properties of aluminum. Its relatively low atomic number and neutron-to-proton ratio contribute to its light weight, malleability, and durability. These properties have made aluminum an indispensable material in countless industries, from aerospace to construction.
Understanding the atomic number of aluminum is not merely an academic exercise; it is a key to unlocking the secrets of this versatile metal. It is a gateway to appreciating its remarkable properties and to harnessing its potential in shaping our world.
Isotopes of Aluminum: Uncovering the Variations of the Element
Aluminum, the third most abundant element in the Earth’s crust, exists in various forms known as isotopes. Each isotope is distinguished by the number of neutrons it contains in its nucleus, resulting in slight variations in their properties.
Aluminum-26, with its unique neutron count of 12, is a relatively rare isotope. Remarkably, this isotope has been found to have applications in nuclear medicine, where it is used as a tracer to diagnose and treat certain conditions.
The most common form of aluminum, aluminum-27, stands out with its 13 neutrons. This isotope forms the core of most aluminum found in our daily lives, contributing to its lightweight and durable characteristics.
Aluminum-28, another notable isotope, possesses 14 neutrons. Its presence is less common compared to its counterparts, but it plays a crucial role in understanding aluminum’s nuclear properties.
These isotopes, although similar in their chemical behavior, exhibit slight differences in their atomic masses due to the varying numbers of neutrons. These differences have implications in various fields, including geochemistry and nuclear physics.
Neutron-to-Proton Ratio in Aluminum: A Balancing Act
Every element in the fascinating realm of science possesses a unique atomic structure, which determines its properties and behavior. One crucial aspect of this structure is the neutron-to-proton ratio, a delicate balance that plays a pivotal role in an element’s stability. In this article, we’ll delve into the intriguing case of aluminum and unravel the significance of its neutron-to-proton ratio.
Understanding the Neutron-to-Proton Ratio
Before exploring aluminum specifically, let’s take a step back and understand what the neutron-to-proton ratio signifies. It refers to the proportion of neutrons to protons within an element’s nucleus. Neutrons, unlike protons, lack electrical charge and contribute to the mass of an atom. The neutron-to-proton ratio influences various aspects, including nuclear stability, radioactive decay, and the element’s position on the periodic table.
Aluminum’s Neutron-to-Proton Ratio: A Delicate Balance
Now, let’s focus on aluminum, an abundant metal renowned for its strength, lightness, and versatility. Aluminum, like all elements, exists in different isotopic forms, each with a distinct number of neutrons. The most prevalent isotope, known as aluminum-27, boasts an equal number of neutrons and protons (13 of each). This remarkable balance results in a neutron-to-proton ratio of approximately 1:1.
The Importance of the 1:1 Ratio
The 1:1 neutron-to-proton ratio in aluminum-27 is not a mere coincidence; it’s essential for the element’s stability and longevity. An imbalance in this ratio can lead to radioactive instability, causing the atom to undergo decay. In the case of aluminum-27, the equal proportion of neutrons and protons creates a stable nucleus, preventing spontaneous radioactive decay.
The neutron-to-proton ratio is a fundamental aspect of an element’s atomic structure, and aluminum-27 serves as a prime example of its profound influence. The 1:1 ratio in aluminum-27 ensures nuclear stability, contributing to the element’s widespread applications in industries ranging from aerospace to construction. As we continue to explore the fascinating world of elements, unraveling the secrets of their atomic structures, we gain invaluable insights into the building blocks of our universe.