Moles Vs. Millimoles: Understanding Substance Quantity In Chemistry

The mole (mol) is a fundamental unit in chemistry representing the amount of substance containing a specific number of elementary entities. The millimole (mmol) is a useful subunit, representing one thousandth of a mole. The conversion factor between these units is 1 mol = 1000 mmol. Millimoles are widely used in measuring smaller quantities of substances, concentration calculations, stoichiometry, and pharmaceutical dosages. Understanding the relationship between moles and millimoles is crucial in various scientific and medical fields for accurate measurement, quantification, and dosage administration.
The Mole: The Fundamental Unit of Measurement
- Explain the concept of the mole as the SI unit for amount of substance.
- Describe the significance of one mole containing a specific number of elementary entities.
The Mole: A Cornerstone of Chemistry
In the realm of chemistry, precise measurements are paramount. The mole, the cornerstone of this field, emerged as a fundamental unit of measurement for amount of substance. This concept revolutionized our understanding of the composition and properties of matter.
One Mole: A Monumental Quantity
A mole represents an awe-inspiring quantity of elementary entities. These entities can be atoms, molecules, ions, or electrons. The International System of Units (SI) defines one mole as the quantity of an element or compound that contains exactly 6.02214076 × 10^23 elementary entities. Remarkably, this number, known as Avogadro’s number, is the foundation of chemistry. It underpins countless calculations, from determining the mass of substances to understanding chemical reactions.
Millimole: A Convenient Subunit
- Define the millimole as one thousandth of a mole.
- Explain the usefulness of millimoles for measuring smaller quantities of substances.
Millimole: A Convenient Subunit for Measuring Smaller Quantities of Substances
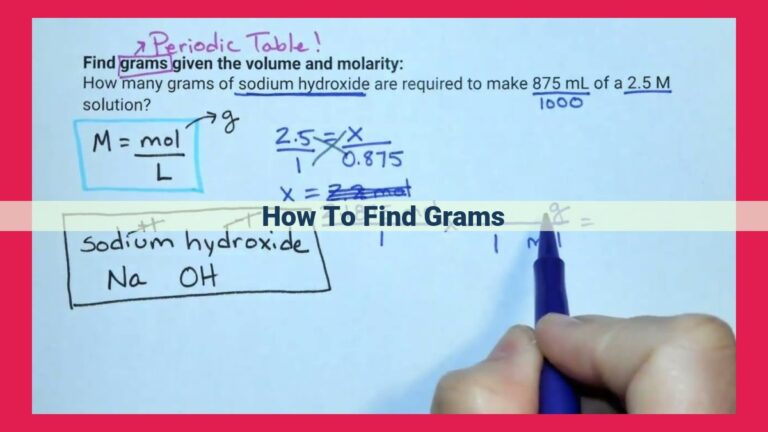
The mole is the fundamental unit of measurement for the amount of substance. It represents a specific number of elementary entities, such as atoms, molecules, or ions. However, for situations involving smaller quantities of substances, the millimole emerges as a useful and convenient subunit.
A millimole is one-thousandth of a mole. It is denoted by the symbol mmol. Millimoles are particularly valuable for measuring smaller quantities of substances, making them more practical in numerous scientific and medical applications.
For example, in concentration calculations, millimoles are commonly used to express the concentration of solutions. By using millimoles per liter (mmol/L), we can accurately determine the amount of substance dissolved in a given volume of solution.
Millimoles also play a crucial role in stoichiometry calculations. Stoichiometry involves determining the quantitative relationships between reactants and products in chemical reactions. By converting quantities to millimoles, we can easily calculate the exact amounts of reactants and products involved in a reaction.
In the realm of pharmacology, millimoles are indispensable for expressing dosages. Precise dosages are essential for ensuring the safe and effective administration of medications. Millimoles allow us to accurately measure and administer the appropriate amount of medication, ensuring optimal efficacy and minimizing the risk of adverse effects.
In summary, the millimole serves as a convenient subunit of the mole, enabling us to measure and quantify smaller quantities of substances with greater precision and ease. Its applications extend across various scientific and medical disciplines, ranging from concentration calculations to stoichiometry and pharmaceutical dosages. Understanding the relationship between moles and millimoles is foundational to navigating these fields with confidence and accuracy.
Conversion Factor: Unveiling the Relationship between Moles and Millimoles
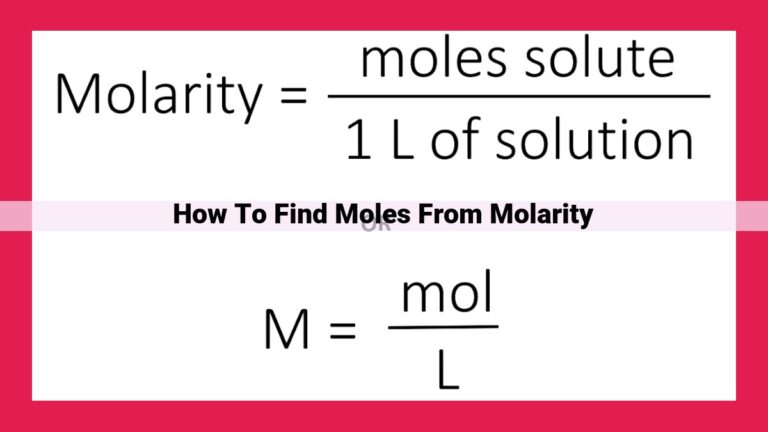
In the scientific world, accurately measuring substances is crucial for various calculations and applications. The mole, the SI unit for amount of substance, represents a specific number of elementary entities, usually atoms or molecules. While the mole is a fundamental concept, it can be inconvenient to work with smaller quantities. This is where the millimole (mmol) comes into play, a subunit of the mole that simplifies measurements.
The Millimole: A Handy Subunit
The millimole is defined as one thousandth of a mole (1 mol = 1000 mmol). It offers a convenient way to express smaller amounts of substances, particularly in fields like chemistry and medicine. For example, in preparing solutions or calculating pharmaceutical dosages, millimoles provide a precise and manageable unit of measurement.
Conversion Factor: A Bridging Tool
The conversion factor serves as a bridge between moles and millimoles, allowing us to seamlessly convert between these units. The relationship is straightforward:
1 mol = 1000 mmol
Example Conversion:
Let’s say we have 0.025 moles of a substance. To convert this to millimoles, we simply multiply by 1000:
0.025 mol × 1000 mmol/mol = 25 mmol
Applications in Science and Medicine
Millimoles play a vital role in various scientific and medical fields:
- Concentration Calculations: In chemistry, millimoles are used to express the concentration of solutions, such as in blood tests or industrial processes.
- Stoichiometry Calculations: Millimoles help determine the amounts of reactants and products involved in chemical reactions, ensuring accurate calculations.
- Pharmaceutical Dosages: In medicine, millimoles are essential for expressing pharmaceutical dosages, ensuring precise and safe administration of medications.
Understanding the relationship between moles and millimoles is crucial for various scientific and medical applications. The conversion factor, 1 mol = 1000 mmol, provides a seamless and accurate way to convert between these units. By grasping this concept, scientists, healthcare professionals, and anyone working with quantities of substances can effectively navigate measurements for precise calculations and informed decision-making.
Real-World Applications of Millimoles
Millimoles, a convenient subunit of the mole, play a crucial role in various scientific and medical applications. Let’s explore some of these practical uses:
Concentration Calculations for Solutions
In chemistry, the concentration of a solution is often expressed in terms of millimoles per liter (mmol/L). This measure indicates the amount of substance present in a specific volume of solution. Millimoles provide a precise way to quantify the concentration, ensuring accurate preparation and analysis of solutions.
Stoichiometry Calculations
Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. By using millimoles, scientists can determine the exact amounts of substances required or produced in a reaction. This knowledge is essential for optimizing reactions, predicting yields, and understanding chemical processes.
Pharmaceutical Dosages
In the realm of medicine, millimoles are vital for expressing pharmaceutical dosages. Accurate drug administration depends on precise measurements of the dosage. Millimoles allow doctors and pharmacists to calculate the appropriate dose for each patient, considering factors such as body weight, age, and condition. This ensures effective and safe treatment.
Understanding the relationship between moles and millimoles is paramount in various scientific and medical fields. The conversion factor of 1 mol = 1000 mmol enables easy interconversion between these units. By comprehending these concepts, researchers, chemists, and healthcare professionals can confidently navigate a wide range of applications, ensuring accuracy and precision in their work.