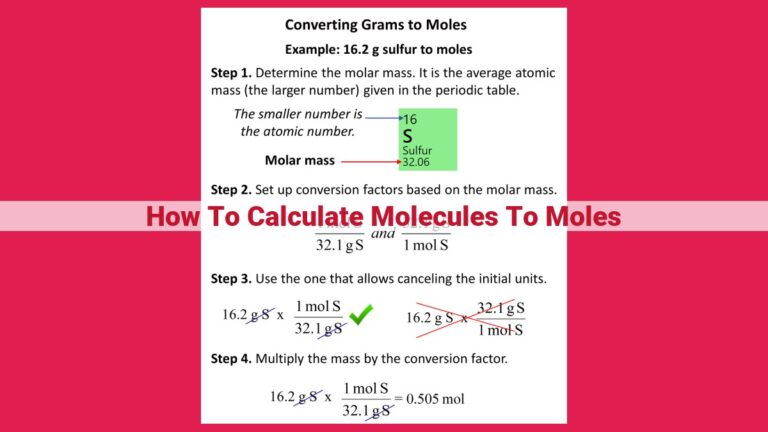
Master The Art Of Converting Moles To Grams: Unlocking Essential Chemistry Principles

Understanding the conversion from moles to grams is crucial in chemistry, biology, and other fields. A mole represents a specific amount of substance, while a gram measures mass. To convert, we use the formula Grams = Moles × Molecular Weight. First, determine the molecular weight by adding the atomic weights of the substance’s atoms. Multiply the number of moles by the molecular weight to obtain the corresponding mass in grams. It’s important to use the correct molecular weight and pay attention to units. Online calculators or reference tables can assist with the conversion.
Converting from Moles to Grams: A Beginner’s Guide
Have you ever wondered how scientists and researchers determine the exact amount of a substance they need for their experiments? It’s through understanding the conversion between moles and grams, two fundamental units of measurement in chemistry. This conversion is like a magical bridge that allows us to translate the number of molecules in a substance to its mass and vice versa.
In this blog post, we’ll embark on a journey to unravel the significance of this conversion and guide you through the steps to effortlessly convert moles to grams. So, grab a cup of coffee or tea, sit back, and let’s dive into the fascinating world of chemistry!
Understanding the Concepts: Moles and Grams
In the realm of chemistry, understanding the conversion between moles and grams is akin to deciphering the secret language of matter. Moles, the units of amount of substance, measure the quantity of a substance based on the number of elementary entities (atoms, molecules, ions, or electrons) it contains.
Just like a baker measures flour by the cup, chemists use moles to quantify the amount of a substance. This concept is particularly significant in stoichiometry, the study of the quantitative relationships between reactants and products in chemical reactions.
Relating Moles to Mass, Weight, and Volume
Moles serve as a bridge between the mass and volume of a substance. Mass refers to the quantity of matter in an object, while weight is the force exerted on an object due to gravity. Grams, the units of mass, are commonly employed in chemistry.
The volume of a substance is the amount of space it occupies. The relationship between moles, mass, and volume depends on the substance’s density. Density, measured in grams per milliliter (g/mL), determines how much mass of a substance is packed into a given volume.
Introducing Grams: The Units of Mass
Complementary to moles, grams are the fundamental units of mass in the metric system. They quantify the amount of matter in an object, regardless of its location or the gravitational force acting upon it. Grams are widely used in weighing solids, liquids, and gases.
Grams, Weight, Density, and Volume
Similar to the relationship between moles and mass, grams are closely tied to weight, density, and volume. Weight, being a measure of the gravitational force acting on mass, is directly proportional to the number of grams in an object.
Density, a substance-specific property, plays a crucial role in relating grams to volume. It determines the mass of a substance per unit volume. Substances with a higher density pack more grams into a smaller volume compared to those with a lower density.
Avogadro’s Number: The Bridge Between Moles and Particles
The connection between moles and particles is made possible by Avogadro’s Number: a constant with a numerical value of approximately 6.022 × 10^23. This number represents the number of elementary entities present in one mole of any substance.
Avogadro’s Number provides a direct conversion factor between moles and the number of particles, enabling chemists to determine the molar mass (mass per mole) of a substance and calculate the number of entities present in a given sample.
Molecular Weight: The Key to Converting Moles to Grams
In the realm of chemistry, the conversion between moles and grams is crucial, and molecular weight plays a pivotal role. Molecular weight, as the name suggests, represents the mass of one mole of a substance. This concept serves as the bridge that connects the world of moles, representing the amount of substance, to the world of grams, representing mass.
To calculate molecular weight, we delve into the microscopic world of atoms. Each atom possesses an atomic weight, a value that indicates its relative mass. By tallying up the atomic weights of all the atoms that make up a molecule, we arrive at the molecular weight of that molecule.
Consider the humble water molecule, H₂O. It comprises two hydrogen atoms and one oxygen atom. The atomic weight of hydrogen is approximately 1, while that of oxygen is approximately 16. Adding these values, we find that the molecular weight of water is approximately 18. This implies that one mole of water molecules has a mass of 18 grams.
The relationship between molecular weight, moles, and mass is a fundamental equation:
Mass = Moles × Molecular Weight
This equation empowers us to seamlessly convert between these units. Understanding molecular weight is key to unlocking the conversion between moles and grams, enabling us to navigate the intricacies of chemical calculations with ease and precision.
Convert Moles to Grams with Ease: A Comprehensive Guide
In the realm of chemistry, understanding the conversion between moles and grams is a fundamental skill. Moles measure the amount of substance, while grams measure mass. This conversion plays a vital role in various fields, including chemistry, biology, and environmental science.
Conversion Formula: The Key to Success
Converting from moles to grams is a straightforward process. The formula you’ll need to master is:
**Grams = Moles × Molecular Weight**
Molecular Weight, simply put, is the mass of one mole of a substance. It’s calculated by adding the atomic weights of all the atoms in the molecule.
Steps for Conversion: A Step-by-Step Guide
To successfully convert moles to grams, follow these steps:
- Identify the Substance: Determine the substance you’re converting.
- Find Molecular Weight: Research the molecular weight of the substance, which is commonly found in reference tables or online databases.
- Plug and Calculate: Substitute the mole value and molecular weight into the formula. The result will be the mass in grams.
Importance of Accuracy: The Right Way to Convert
Accuracy is paramount when converting moles to grams. Using the correct molecular weight for the specific substance is crucial. A wrong molecular weight will lead to an inaccurate conversion.
Example: A Practical Illustration
Let’s say you have 0.5 moles of glucose (C6H12O6). The molecular weight of glucose is 180.16 g/mol.
- Step 1: Identify the substance: Glucose (C6H12O6)
- Step 2: Find molecular weight: 180.16 g/mol
- Step 3: Convert: 0.5 moles × 180.16 g/mol = 90.08 g
Hence, 0.5 moles of glucose is equivalent to 90.08 grams.
Converting Moles to Grams: A Comprehensive Guide
Understanding the conversion between moles and grams is crucial in various scientific disciplines. This blog post will delve into the concepts behind this conversion and provide a step-by-step guide to help you master it.
Understanding the Concepts
A mole is a unit of measurement for the amount of substance. It represents a specific number of particles, known as Avogadro’s Number, which is 6.022 × 10^23. Conversely, a gram is a unit of measurement for mass.
The relationship between moles and grams is determined by the substance’s molecular weight. Molecular weight is the mass of one mole of that substance, expressed in grams per mole (g/mol).
Molecular Weight
To calculate the molecular weight of a substance, sum the atomic weights of each element present in its molecular formula. For example, the molecular weight of water (H2O) is:
2 × atomic weight of Hydrogen (1 g/mol) + 1 × atomic weight of Oxygen (16 g/mol) = 18 g/mol
Conversion Process
The formula for converting moles to grams is:
Grams = Moles × Molecular Weight
To convert moles to grams, follow these steps:
- Identify the substance: Determine the substance for which you need to convert moles to grams.
- Find the molecular weight: Look up the molecular weight of the substance in a periodic table or reference table.
- Substitute values: Multiply the number of moles by the molecular weight. The result will be the mass in grams.
Example Conversion
Let’s convert 2 moles of sodium chloride (NaCl) to grams using the formula:
- Molecular weight of NaCl: 22.99 + 35.45 = 58.44 g/mol
- Conversion: 2 moles × 58.44 g/mol = 116.88 grams
Tips for Conversion
- Verify molecular weight: Ensure you use the correct molecular weight for the specific substance.
- Pay attention to units: Multiply values with consistent units to avoid errors.
- Use calculators or tables: Online calculators and reference tables can provide convenience and accuracy.
Understanding the conversion between moles and grams is essential in various fields. By applying the concepts and formula presented in this guide, you can confidently convert between these units and accurately calculate masses of substances. Remember to pay attention to units and verify the molecular weight for precise conversions.
Converting Moles to Grams: A Guide for Students and Researchers
Understanding the Concepts
- Mole: A mole is the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
- Gram: A gram is a base unit of mass in the metric system.
- Avogadro’s Number: 6.022 × 10^23 particles are present in one mole of any substance.
Molecular Weight
- Molecular weight is the mass of one mole of a substance.
- Calculated by adding the atomic weights of the atoms in the molecule.
Conversion Process
- Formula: Grams = Moles × Molecular Weight
- Steps:
- Determine the molecular weight of the substance.
- Multiply the number of moles by the molecular weight.
Example Conversion
- Convert 2.5 moles of sodium chloride (NaCl) to grams.
- Molecular weight of NaCl: 58.44 g/mol
- Calculation: Grams = 2.5 moles × 58.44 g/mol = 146.1 g
Tips for Conversion
- Verify the molecular weight you are using.
- Pay attention to units when multiplying to ensure consistency.
- Use online calculators or reference tables for convenience and accuracy.