Molecule’s Chemical Formula Missing In Provided Context: Delving Into Structural And Bonding Concepts
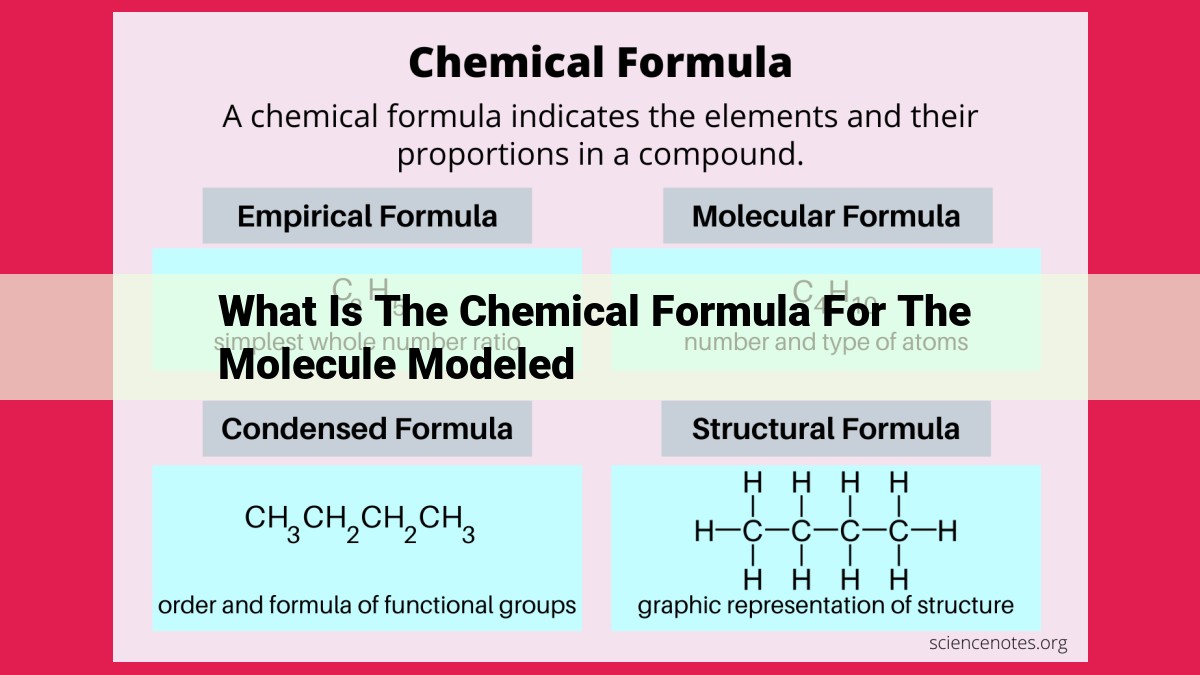
The chemical formula for the modeled molecule is not provided in the given context. The provided text focuses on concepts related to molecular structure, chemical composition, formula calculations, chemical bonding, and spectroscopic data. It does not include specific information about the chemical formula of a particular molecule.
Unveiling the Secrets of Molecular Structure
In the realm of chemistry, understanding molecular structure holds the key to comprehending the properties and behaviors of chemicals that shape our world. Molecular structure refers to the arrangement of atoms within a molecule, and it profoundly influences everything from a substance’s chemical reactivity to its physical state.
Delving into Molecular Geometry and Molecular Weight
Molecular geometry, intertwined with molecular structure, describes the three-dimensional shape of molecules. This shape plays a crucial role in determining the molecule’s polarity, reactivity, and even its biological functions. For instance, linear molecules, such as carbon dioxide, display different properties compared to bent molecules, like water.
Another essential aspect of molecular structure is molecular weight. It represents the mass of a molecule relative to the mass of a single carbon atom. Molecular weight serves as a valuable tool in identifying and characterizing molecules, especially in the analysis of complex mixtures.
As we delve into the fascinating world of molecular structure, we uncover a tapestry of interconnected concepts that paint a deeper understanding of the chemical world around us.
**Understanding Molecular Composition**
Every molecule in the vast tapestry of our universe possesses a unique molecular composition, a fingerprint that reveals its identity. This composition defines the building blocks of the molecule and provides invaluable insights into its properties and behavior.
**Chemical Composition: The Essence of Molecules**
Chemical composition refers to the types and proportions of atoms that make up a molecule. It provides a fundamental understanding of the molecule’s elemental makeup. Knowing a molecule’s chemical composition enables scientists to discern its chemical formula, a shorthand notation that concisely represents its atomic constituents.
**Empirical Formula vs. Molecular Formula: Unveiling Precision**
Scientists employ two distinct types of chemical formulas to characterize molecular composition: empirical formula and molecular formula. The empirical formula reveals the simplest whole-number ratio of atoms present in a molecule. It provides a rudimentary understanding of the molecule’s composition, focusing on the atomic ratios rather than the exact number of atoms.
In contrast, the molecular formula pinpoints the exact number of atoms of each element present in a molecule. It is a more precise representation of the molecule’s composition, providing detailed insights into its atomic structure.
**Bridging the Gap: Empirical Formula to Molecular Formula**
The empirical formula and molecular formula are two sides of the same coin, each offering a unique perspective on a molecule’s composition. The empirical formula can be particularly useful when working with complex molecules where determining the molecular formula may be challenging. By understanding the relationship between the empirical formula and molecular formula, scientists can gain a deeper understanding of the molecule’s structure and bonding arrangements.
Delving into Empirical Formula and Molecular Formula
In the realm of chemistry, understanding the composition of molecules is as crucial as unraveling their structure. Two fundamental concepts that aid in this pursuit are empirical formula and molecular formula.
Empirical Formula: A Window into the Simplest Atomic Ratio
Imagine a molecule as a miniature world of atoms, each contributing its share to its identity. The empirical formula of a compound provides a glimpse into its simplest whole-number ratio of elements. It reveals the relative proportions of different atoms, like a blueprint outlining the molecular framework.
Calculating an empirical formula involves determining the masses of each element present in a compound and then dividing those masses by their respective atomic masses. The resulting ratios are expressed as the simplest possible whole numbers.
Molecular Formula: Unveiling the Exact Atomic Composition
While the empirical formula provides a snapshot of the atomic ratio, the molecular formula goes a step further. It reveals the exact number of atoms of each element in a molecule, giving us a precise blueprint of its atomic composition.
Determining the molecular formula requires knowing the empirical formula and the molar mass of the compound. The molar mass is the sum of the atomic masses of all the atoms in the molecule. By dividing the molar mass by the mass represented by the empirical formula, we obtain a number that indicates how many times the empirical formula must be multiplied to arrive at the molecular formula.
The Intricate Relationship between Empirical and Molecular Formula
The empirical formula and molecular formula are two sides of the same coin. The empirical formula offers a simplified view of the atomic composition, while the molecular formula provides the complete picture. For instance, the empirical formula of glucose is CH2O, indicating a 1:2:1 ratio of carbon, hydrogen, and oxygen atoms. However, its molecular formula, C6H12O6, reveals that glucose is composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
Understanding the interplay between these two formulas is crucial for unraveling the true nature of molecules. They provide a foundation for further exploration, aiding chemists in elucidating molecular structures and properties.
Unraveling Chemical Bonding: The Invisible Forces Holding Atoms Together
In the intricate world of molecules, chemical bonding plays a pivotal role, acting as the invisible glue that holds atoms in place. Without this fundamental force, molecules would simply disintegrate into their constituent parts.
Types of Chemical Bonds
Chemical bonds come in various forms, each with its unique characteristics:
- Covalent Bonds: In covalent bonding, atoms share electrons to create a stable molecule. These bonds are typically found in nonmetals, such as in the covalent bond between two hydrogen atoms in H2.
- Ionic Bonds: When atoms transfer electrons from one atom to another, they form ionic bonds. This type of bond occurs between metals and nonmetals, such as in the ionic bond between sodium (Na) and chlorine (Cl) in NaCl.
- Metallic Bonds: Metallic bonds are formed when metal atoms share their valence electrons in a “sea of electrons.” This type of bonding is responsible for the unique properties of metals, including their high electrical and thermal conductivity.
Influence of Chemical Bonding on Molecular Structure
The type of chemical bond present significantly influences the molecular structure. Covalent bonds lead to molecules with specific geometries, such as the tetrahedral shape of methane (CH4) or the linear shape of carbon dioxide (CO2). Ionic bonds, on the other hand, form crystalline solids with highly organized structures, like the cubic structure of sodium chloride.
Relationship between Chemical Bonding and Molecular Geometry
Chemical bonding and molecular geometry are deeply intertwined. The spatial arrangement of atoms within a molecule is determined by the types of bonds present. For example, the tetrahedral shape of methane arises from the four covalent bonds formed between the carbon atom and the four hydrogen atoms.
Understanding chemical bonding is crucial for deciphering the behavior and properties of molecules. It provides insights into their stability, reactivity, and various applications in chemistry and beyond.
Unveiling the Secrets of Molecules: Leveraging Spectroscopic Data
In the quest to unravel the intricate nature of molecules, scientists have harnessed a powerful tool: spectroscopic data. This data provides invaluable insights into the molecular structure and composition that shape the world around us.
Defining Spectroscopic Data
Spectroscopic data is a record of the interaction between light or other forms of electromagnetic radiation with molecules. This interaction results in the absorption or emission of energy, which provides a unique fingerprint for each molecule. By analyzing these energy changes, scientists can deduce the molecular structure and composition.
Obtaining Spectroscopic Data
Spectroscopic data is obtained using spectroscopic techniques, which are based on the principle of shining specific wavelengths of light or other radiation at the sample and measuring the resulting response. Common spectroscopic techniques include:
- Ultraviolet-Visible (UV-Vis) Spectroscopy: Analyzes the absorption of light in the ultraviolet and visible regions, providing information about electronic structure and conjugation.
- Infrared (IR) Spectroscopy: Detects the absorption of infrared radiation, revealing information about functional groups and molecular vibrations.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Uses radio waves to determine the structure and dynamics of molecules, providing insights into atomic connectivity and molecular motion.
Significance of Spectroscopic Data
Spectroscopic data is crucial for understanding molecular structure and composition because:
- It provides fingerprint-like information that can be used to identify and differentiate molecules.
- It allows scientists to determine the arrangement of atoms within molecules, known as molecular geometry.
- It reveals the presence of specific functional groups, which are responsible for the chemical properties of molecules.
- It enables the investigation of molecular dynamics and interactions, providing insights into reaction mechanisms and biological processes.
Applications of Spectroscopic Techniques
Spectroscopic techniques find wide applications in various fields, including:
- Chemistry: Identifying and characterizing organic and inorganic compounds, studying reaction mechanisms, and analyzing molecular structure.
- Biology: Determining the structure and dynamics of proteins, nucleic acids, and other biomolecules, and investigating biological processes.
- Medicine: Diagnosing diseases, monitoring drug efficacy, and studying metabolic pathways.
- Environmental Science: Analyzing air and water pollution, detecting contaminants, and monitoring ecosystem health.
Spectroscopic data is a priceless tool that empowers scientists to probe the molecular realm. By leveraging spectroscopic techniques, researchers can unravel the complexities of molecular structure and composition, unlocking new discoveries and fostering advancements in chemistry, biology, medicine, and beyond.