Factors Influencing Molecular Diversity: A Key To Unlocking Chemical Complexity
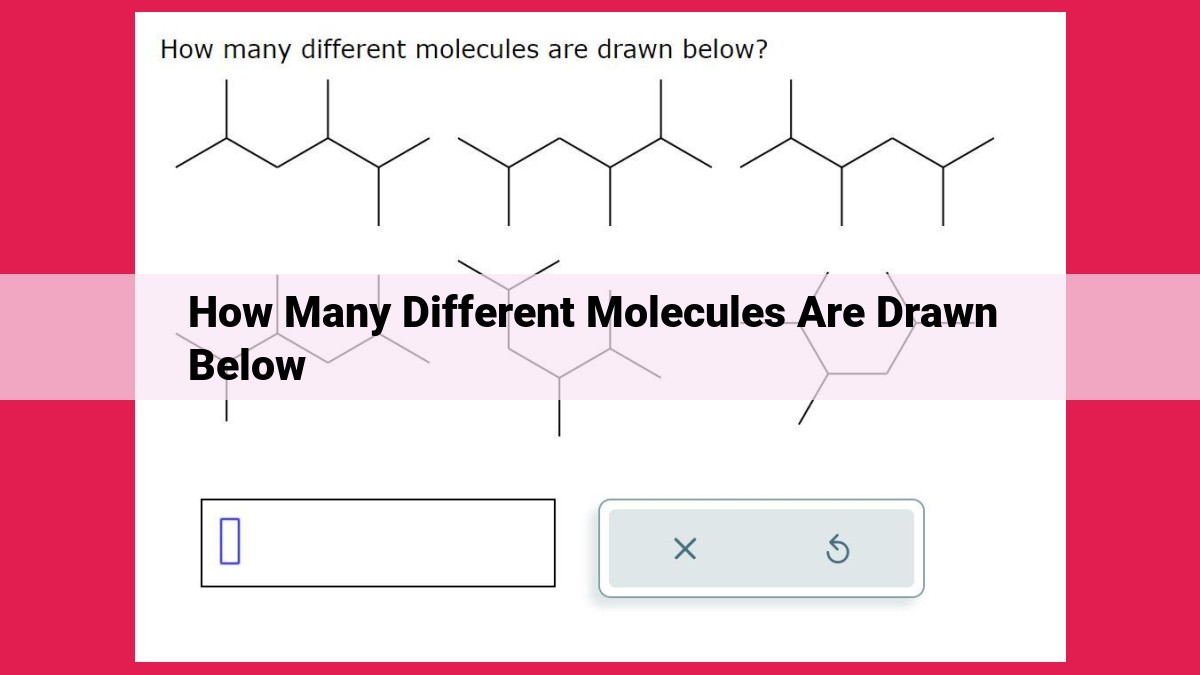
Understanding factors that influence molecular diversity is crucial when drawing molecules. Different factors contribute to this diversity, including structural isomers, functional groups, resonance, and chirality. Functional groups influence molecular properties and reactivity, while resonance affects stability and reactivity by electron delocalization. Chirality adds complexity with optical activity and its significance in biological systems. Considering these factors helps predict the number of distinct molecules that can be drawn for a given formula, showcasing the vast chemical diversity in nature.
Molecular Diversity: A Key to Understanding the Chemical World
In the vast tapestry of the chemical world, molecules dance and interact in an intricate ballet, exhibiting a kaleidoscope of diversity that confounds and captivates scientists alike. At the heart of this diversity lies a myriad of factors that govern the countless molecular structures that can be drawn for a given molecular formula. Understanding these factors is paramount for chemists seeking to predict, synthesize, and understand the chemical interactions that shape our world.
Factors Influencing Molecular Diversity:
- Number of Isomers: Molecules with the same molecular formula can have different structures, known as isomers. Constitutional isomers differ in the arrangement of atoms, while stereoisomers differ in the spatial arrangement of atoms. The number of isomers increases exponentially with the complexity of the molecule.
- Functional Groups: Functional groups are specific groups of atoms that impart characteristic properties and reactivities to molecules. The nature and number of functional groups significantly impact molecular diversity.
- Resonance: Resonance is a concept that describes the delocalization of electrons within a molecule, resulting in multiple possible Lewis structures. This phenomenon stabilizes molecules, affects their reactivities, and expands the range of possible molecular structures.
- Chirality: Chirality refers to the presence of asymmetry in a molecule, resulting in two non-superimposable mirror images called enantiomers. Chirality is crucial in biological systems, where enantiomers can have vastly different biological activities.
Factors Determining Molecular Diversity
In the realm of chemistry, understanding the factors that influence molecular diversity is crucial for predicting the vast array of molecules that can be formed. These factors play a pivotal role in determining the unique properties and behaviors of each molecule.
Number of Different Structural Isomers (Constitutional and Stereoisomers)
Structural isomers are molecules with the same molecular formula but different structural arrangements. Constitutional isomers have different covalent arrangements of atoms, while stereoisomers have the same covalent connections but differ in their spatial orientation. For instance, butane has two constitutional isomers (n-butane and isobutane) and three stereoisomers (two enantiomers and one meso compound).
Different Functional Groups and Their Interconversions
Functional groups are specific arrangements of atoms within a molecule that impart characteristic chemical properties. Different functional groups, such as alcohols, aldehydes, and ketones, exhibit distinct reactivities and can undergo interconversions to form a wide variety of molecules. For example, an alcohol can be oxidized to an aldehyde, which can then be further oxidized to a ketone.
Number of Possible Resonance Structures
Resonance occurs when a molecule has multiple valid Lewis structures due to the delocalization of electrons over several atoms. The number of possible resonance structures contributes to the overall stability and reactivity of the molecule. Benzene, with its six resonance structures, is a classic example of a highly resonance-stabilized molecule.
Chirality (Enantiomers and Diastereomers)
Chirality refers to the handedness of a molecule, where non-superimposable mirror images exist. Enantiomers are pairs of chiral molecules that are mirror images of each other, while diastereomers are chiral molecules that are not mirror images. Chirality can profoundly impact biological activity, as many enzymes and receptors are chiral and recognize only one enantiomer of a molecule.
Impact of Functional Groups on Molecular Diversity
Functional groups, the building blocks of organic molecules, play a pivotal role in defining their properties and reactivity. They introduce new chemical functionalities, altering the chemical landscape of a molecule and unlocking a cascade of possibilities for further transformations.
Chemical Properties and Reactivity
Functional groups dictate the chemical behavior of molecules. For instance, the presence of hydroxyl (-OH) groups imparts polarity and hydrogen bonding capabilities, making molecules more soluble in water. In contrast, carbonyl (C=O) groups possess an electrophilic carbon atom, prone to nucleophilic attack and subsequent chemical reactions.
Transforming Functional Groups
Chemical reactions can interconvert functional groups, creating new molecular diversity. For example, oxidation of a primary alcohol converts it to an aldehyde, while reduction of a ketone yields a secondary alcohol. These transformations open up new avenues for further chemical modifications.
Applications in Synthesis
The ability to transform functional groups is critical in organic synthesis. By manipulating functional groups, chemists can create complex and tailor-made molecules with desired properties. This versatility enables the development of new pharmaceuticals, materials, and advanced technologies.
The Magical Dance of Resonance: Unlocking Molecular Diversity
Have you ever wondered why certain molecules exude stability, while others are highly reactive? One of the secret ingredients behind this molecular diversity is the captivating concept of resonance. In this blog post, we’ll embark on a storytelling journey to unravel the enchanting dance of resonance and its profound impact on molecular properties.
What is Resonance?
Picture resonance as a molecular waltz, where electrons twirl and embrace in a harmonious dance. This electron ballet results in multiple resonance structures, each representing a different arrangement of double bonds and lone pairs. These structures are not independent entities but rather interconvertible through the smooth flow of electrons.
Stability and Reactivity: The Resonance Balancing Act
The dance of resonance has a profound impact on molecular stability and reactivity. By delocalizing electrons, resonance creates a more stable molecule as the electrons are spread over a larger area. This electron delocalization also reduces the reactivity of the molecule by dispersing its electronic charge. As a result, resonance stabilizes molecules and makes them less prone to reactions.
Molecular Structure: The Shape of Resonance
The resonance dance not only influences stability but also shapes molecular structure. The delocalization of electrons through resonance results in a more uniform distribution of electron density, which leads to a symmetrical molecular geometry. This symmetry often manifests in a planar or linear molecular structure, enhancing the stability and minimizing energy levels.
Resonance in Action: Benzene’s Aromatic Stability
Let’s take benzene as a prime example of resonance’s transformative power. Benzene has a ring structure with alternating double and single bonds, giving rise to two possible resonance structures. The resonance dance between these structures delocalizes the electrons, creating a ring of aromatic stability that makes benzene highly resistant to chemical reactions.
Resonance is an enchanting dance that profoundly influences molecular diversity. By delocalizing electrons, it enhances stability, reduces reactivity, and shapes molecular structure. This mesmerizing interplay of electrons is a testament to the intricate nature of chemical bonding and the vast chemical world it opens up for our exploration.
Role of Chirality in Molecular Diversity
In the enchanting realm of chemistry, chirality unveils a fascinating aspect of molecular diversity. Chirality refers to the asymmetry of a molecule, a property that endows it with a mirror-image counterpart but prevents them from being superimposed upon one another. This seemingly subtle difference leads to profound implications in molecular properties and biological significance.
Chirality and Molecular Properties
Chiral molecules exhibit unique physical and chemical attributes. Optical activity, a distinguishing hallmark of chirality, describes the ability of molecules to interact differently with polarized light. When light passes through a chiral substance, its plane of polarization undergoes a characteristic rotation. The magnitude and direction of this rotation depend on the specific chiral structure.
Importance in Biological Systems
Chirality plays a pivotal role in biological systems. Enzymes, the catalysts that drive countless cellular reactions, display exquisite selectivity for specific chiral substrates. This specificity ensures the proper functioning and regulation of metabolic pathways. Likewise, the chirality of DNA and proteins determines their biological activity and recognition by cellular machinery.
Examples and Applications
The impact of chirality extends far beyond the laboratory. Pharmaceuticals often comprise chiral molecules, and their different enantiomers can exhibit vastly different pharmacological properties. Natural products, such as terpenes and alkaloids, frequently display chirality, contributing to their diverse biological functions. Understanding chirality is thus crucial for developing effective drugs, synthesizing natural compounds, and unlocking the secrets of life itself.
In conclusion, the concept of chirality opens up a captivating chapter in molecular diversity. Its influence on molecular properties and biological systems underscores the intricate interplay between structural asymmetry and the boundless possibilities of chemical creation. By unraveling the enigmas of chirality, we delve deeper into the fundamental building blocks of our world and gain a profound appreciation for the astonishing diversity of nature’s molecular tapestry.