Optimize Title For Seo:molarity: A Comprehensive Guide For Measuring Solution Concentration
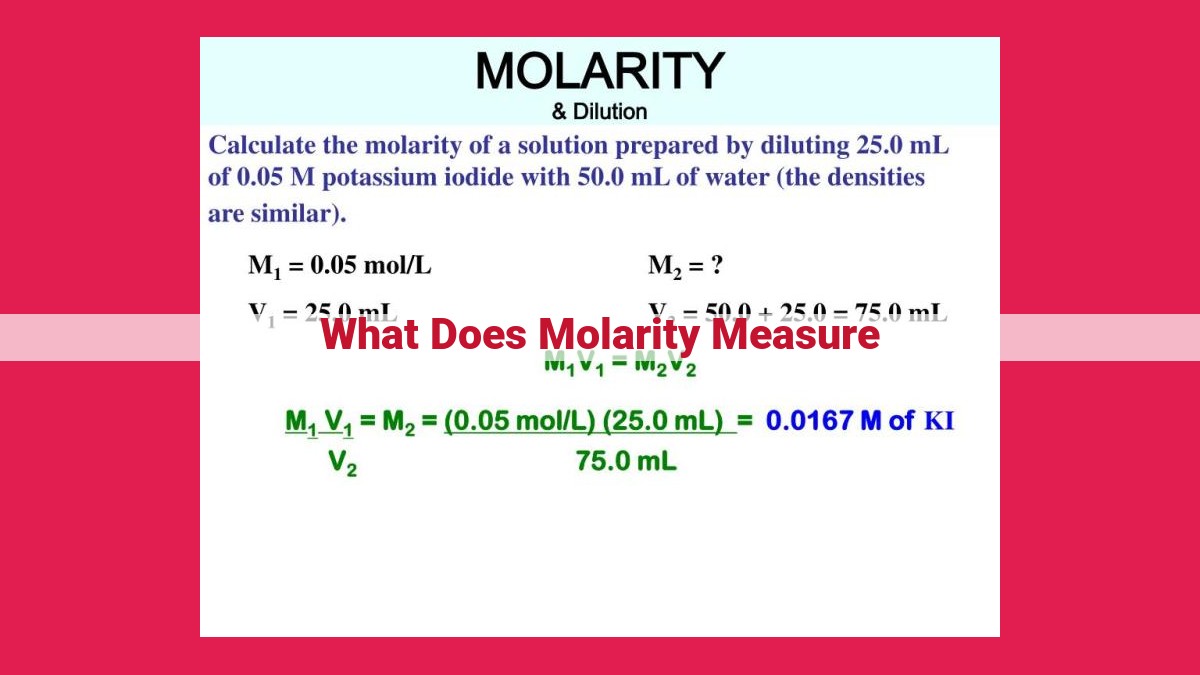
Molarity is a measure of the concentration of a solution, specifically the number of moles of solute (the substance being dissolved) per liter of solution. It’s used to quantify the number of moles of solute in a given volume. Molarity is an important concept in chemistry as it allows scientists to accurately determine the concentration of solutions, facilitating the precise manipulation and analysis of solutions in various scientific and industrial applications.
Understanding Molarity: A Metric for Concentration
In the world of chemistry, the concept of concentration is crucial for understanding the behavior of solutions. Molarity, a key measure of concentration, quantifies the amount of solute dissolved in a solvent. In this blog post, we’ll delve into the concept of molarity and its significance in various fields of science and industry.
Defining Molarity
Molarity is a standardized measure of concentration that expresses the number of moles of a solute per liter of solution. A mole is the SI unit for the amount of substance, representing a specific quantity of particles (atoms, molecules, or ions). By dividing the number of moles by the volume of the solution in liters, we obtain the molarity (M) of the solution.
Importance of Molarity
Molarity plays a fundamental role in chemistry as it allows scientists to precisely determine the amount of solute present in a given solution. This information is critical for:
- Conducting chemical reactions
- Analyzing the properties of solutions
- Comparing the concentrations of different solutions
Essential Related Concepts for Understanding Molarity
To comprehend the concept of molarity, it’s crucial to grasp the fundamentals of related concepts like the mole, solute, and solvent.
a) The Mole: Measuring the Amount of Substance
The mole (Symbol: mol) is the SI unit used to quantify the amount of substance. It represents a specific number of particles, similar to how a dozen (12) signifies a specific number of objects. Precisely, one mole is defined as the quantity of a substance that contains exactly 6.022 x 10^23 entities, which can be atoms, molecules, ions, or electrons. This vast number, known as Avogadro’s number, serves as the foundation for determining the amount of solute in a solution.
b) The Solute: The Dissolved Substance
Molarity measures the concentration of a solute, the substance that dissolves in a solvent to form a solution. The solute and solvent together constitute the solution. The amount of solute is typically expressed in moles. For example, in a 1 M (1 molar) solution of sodium chloride (NaCl), there is 1 mole of dissolved sodium chloride for every 1 liter of solution.
c) The Solvent: The Dissolving Medium
The solvent is the liquid in which the solute dissolves, forming a homogeneous mixture. The solvent plays a crucial role in determining the solute’s solubility and behavior. A good solvent readily dissolves the solute and allows it to disperse evenly throughout the solution. Common solvents include water, alcohol, and various organic liquids.
Understanding these concepts provides a solid foundation for comprehending molarity, a fundamental measure of concentration that enables chemists to accurately quantify and manipulate solutions for various scientific and industrial applications.
Concentration Measures and the Precision of Molarity
Concentration, the cornerstone of chemistry, refers to the amount of a particular solute dissolved within a solvent. Understanding how to accurately measure this concentration is crucial for various scientific and industrial applications.
Amongst the different concentration measures, molarity stands out as the most precise and reliable. Molarity, denoted by the symbol M, quantifies the number of moles of solute present in one liter of solution. It’s a standardized measure that empowers chemists to determine the concentration of solutions with unparalleled accuracy.
The mole, the SI unit for amount of substance, represents a specific quantity of particles. One mole of any substance contains 6.022 × 10^(23) particles, known as Avogadro’s number. This concept enables chemists to precisely calculate the mass of solute needed to prepare a solution of a specific molarity.
To determine the molarity of a solution, we simply divide the number of moles of solute by the volume of the solution in liters. This simple yet powerful formula provides a direct and accurate measure of the concentration of the solution. Understanding molarity eliminates guesswork and ensures precision in preparing and analyzing solutions, making it an indispensable tool for chemists.
Applications and Importance of Molarity
Unveiling the Power of Molarity: From the Lab to the Factory Floor
Molarity, the measure of a solution’s concentration, plays a pivotal role in scientific research and industrial processes alike. Its precision and versatility have made it indispensable in diverse fields, from chemistry and medicine to manufacturing and agriculture.
Scientific Precision: Unveiling the Secrets of Solutions
In the realm of science, molarity empowers chemists to conduct precise experiments. By accurately determining the concentration of reactants and products, molarity allows them to:
- Quantify chemical reactions: Measure the exact amounts of substances that undergo chemical change.
- Study equilibrium: Determine the concentrations at which reactions reach a balance.
- Analyze complex mixtures: Identify and quantify different components within a solution.
Industrial Applications: Harnessing Solutions’ Potential
In the industrial sector, molarity is equally crucial. It enables manufacturers to optimize processes, ensure product quality, and control environmental impact:
- Chemical production: Control the concentration of reactants to produce specific products with desired properties.
- Water treatment: Measure the concentration of impurities and adjust treatment methods to ensure clean water.
- Fertilizer production: Determine the optimal concentration of nutrients for specific crop requirements.
Examples: Seeing Molarity in Action
The versatility of molarity is further exemplified by its applications in various fields:
- Medicine: Calculate the molarity of drugs to ensure proper dosage and treatment efficacy.
- Environmental science: Monitor the concentration of pollutants in water and soil to assess environmental impact.
- Food science: Measure the molarity of acids in fruits to determine ripeness and adjust acidity in beverages.
Molarity stands as the cornerstone of chemistry, providing a standardized measure for solution concentration. Its applications extend far beyond the laboratory, empowering scientists and industrialists to analyze, manipulate, and understand the behavior of solutions. As a tool for precision and optimization, molarity continues to play an indispensable role in advancing scientific knowledge and driving industrial progress.