Molar Mass Of Sucrose: Definition, Calculation, And Role In Stoichiometry
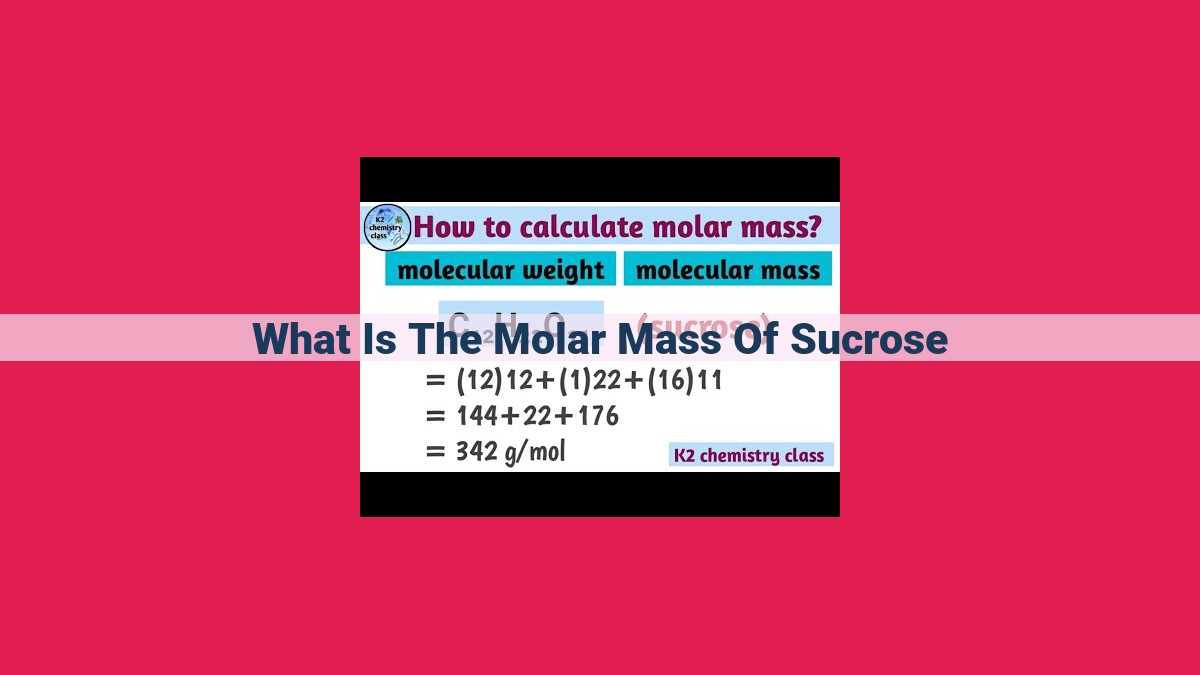
The molar mass of sucrose, C₁₂H₂₂O₁₁, is 342.3 g/mol. It represents the mass of 6.022 × 10²³ molecules of sucrose. The molar mass is calculated by summing the atomic masses of its constituent elements: carbon (12 × 12.01 g/mol), hydrogen (22 × 1.01 g/mol), and oxygen (11 × 16.00 g/mol). Molar mass plays a vital role in stoichiometry, enabling the conversion between mass and moles of a substance, which is crucial in various chemical reactions and quantitative analysis.
Delving into Molar Mass: The Key to Understanding Sucrose
Molar mass is a scientific concept that’s essential for understanding the composition and properties of substances like sucrose. It represents the mass of one mole of a molecule or compound, which is an incredibly large number of particles. In the world of chemistry, the mole is the standard unit for measuring the amount of a substance.
Sucrose is a common sugar found in many foods. It’s a disaccharide, meaning it consists of two sugar molecules (glucose and fructose) linked together. Sucrose plays a vital role in providing energy and sweetness to our bodies.
Now, let’s dive into the components of molar mass to understand how we can calculate the molar mass of sucrose.
Components of Molar Mass: Building Blocks of Chemical Calculations
In the realm of chemistry, understanding the concept of molar mass is paramount. It serves as the cornerstone for various calculations and conversions, enabling us to delve into the intricate world of matter. To comprehend molar mass, we must unravel the fundamental components that contribute to its essence. Let’s embark on this adventure, dissecting each element and exploring its significance.
Atomic Mass: The Essence of Elements
At the heart of molar mass lies atomic mass, the intrinsic property of individual atoms. It represents the mass associated with a single atom of an element, providing a quantitative measure of its substance. Imagine each atom as a minuscule building block, with its atomic mass reflecting the weight of its constituent particles, protons, and neutrons. For instance, the atomic mass of carbon, a crucial element in organic chemistry, is approximately 12 atomic mass units (amu).
Molecular Mass: A Union of Atoms
As atoms combine to form molecules, their collective mass is captured by molecular mass. This concept extends beyond individual atoms, encompassing the aggregate mass of all atoms within a molecule. Consider the familiar molecule of water (H₂O). Its molecular mass is calculated by summing the atomic masses of two hydrogen atoms and one oxygen atom, equaling approximately 18 amu. Molecular mass thus mirrors the cumulative heft of the constituent atoms, providing a quantitative representation of the molecule as a whole.
Mole: The Counting Unit of Chemistry
In the realm of chemistry, we encounter the concept of a mole, a fundamental unit for counting entities. A mole represents a specific number of particles, akin to a dozen eggs or a gross of pencils. In the context of molar mass, a mole corresponds to 6.022 × 10²³ particles, an immense quantity known as Avogadro’s number. This numerical value serves as a universal conversion factor, enabling us to effortlessly translate between mass and the number of particles present.
Calculating the Molar Mass of Sucrose: A Journey to Understanding Sweetness
When it comes to the delectable sweetness of sucrose, understanding its molar mass is crucial. This value represents the mass of one mole of a substance, which is the basic unit for measuring the amount of material present. For sucrose, a beloved component in many of our culinary delights, we embark on a journey to uncover its molar mass and its significance in the world of chemistry.
To delve into this concept, we must first understand its components. As sucrose is a compound, it consists of multiple elements. The atomic mass of each element, representing the mass of a single atom, plays a vital role in determining the molar mass. Carbon, hydrogen, and oxygen, the building blocks of sucrose, have atomic masses of 12.011 g/mol, 1.008 g/mol, and 16.000 g/mol, respectively.
The formula of sucrose provides a blueprint for its composition: C₁₂H₂₂O₁₁. This formula indicates that each sucrose molecule is composed of 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms. To calculate the molar mass, we multiply the atomic mass of each element by its respective number of atoms and then sum these values.
For sucrose, the calculation is as follows:
Molar Mass of Sucrose = (12 × 12.011 g/mol) + (22 × 1.008 g/mol) + (11 × 16.000 g/mol)
Molar Mass of Sucrose = 144.132 g/mol + 22.176 g/mol + 176.000 g/mol
Molar Mass of Sucrose = 342.308 g/mol
Therefore, the molar mass of sucrose is 342.308 g/mol, signifying the mass of one mole of sucrose. This value serves as a cornerstone for understanding the substance’s properties and its behavior in chemical reactions.
The Significance of Molar Mass: A Deeper Understanding
In the realm of chemistry, the concept of molar mass plays a crucial role in quantitating substances and comprehending chemical reactions. Molar mass, expressed in grams per mole (g/mol), represents the mass of one mole of a compound.
Use in Stoichiometric Calculations
Stoichiometry, the art of balancing chemical equations, relies heavily on molar mass. By determining the molar mass of reactants and products, chemists can calculate the exact amounts of substances involved in a reaction. This knowledge enables them to predict the quantities of products formed or the amount of reactants required.
Conversion between Mass and Moles of a Substance
Molar mass serves as a bridge between the mass and the number of moles of a substance. Given the mass of a compound, its molar mass allows us to calculate the corresponding number of moles using the formula:
Number of Moles = Mass (g) / Molar Mass (g/mol)
Conversely, if the number of moles is known, the mass can be determined by multiplying the moles by the molar mass. This conversion is essential in practical applications, such as preparing solutions or analyzing experimental data.
In summary, molar mass is a fundamental concept in chemistry. It empowers chemists to understand stoichiometry, convert between mass and moles, and make quantitative predictions in chemical reactions. By delving into the significance of molar mass, we gain a deeper appreciation for its role in advancing our understanding of the chemical world.