Determining Molar Mass: A Guide To Calculating Substance Mass
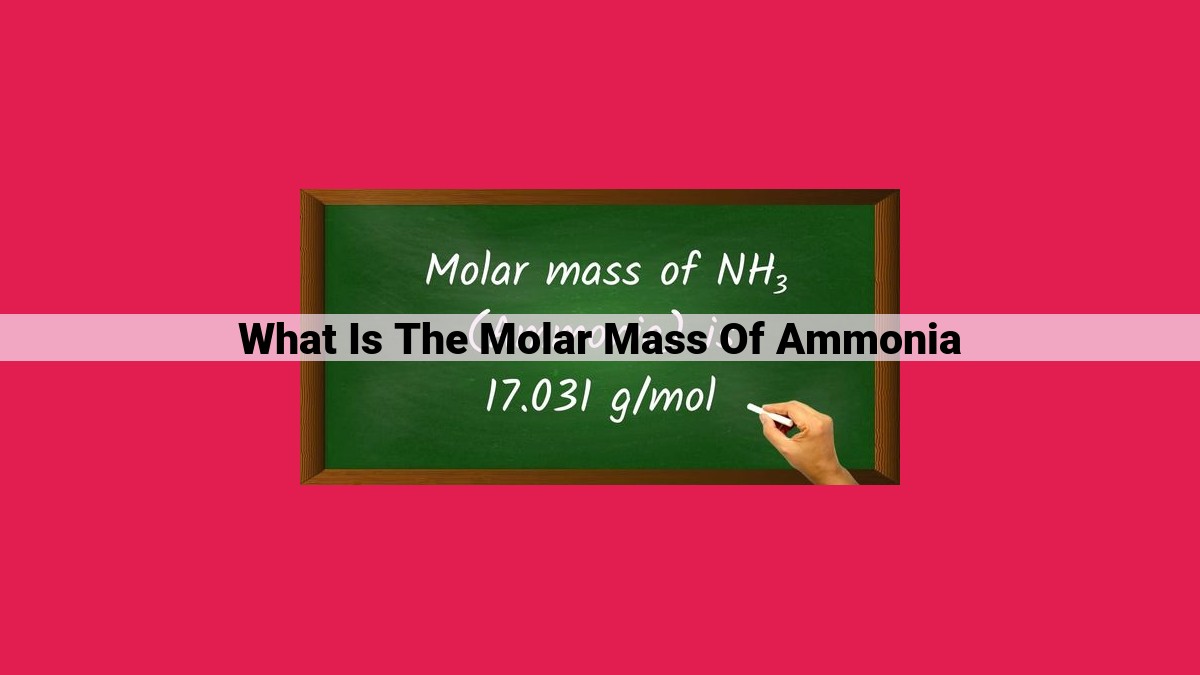
Molar mass, expressed in grams per mole, represents the mass of one mole of a substance. To calculate molar mass, we multiply the atomic mass of each element in a compound by its number of atoms and sum these values. Using the periodic table, we can determine atomic masses based on atomic numbers and observe periodic trends in molar mass. For ammonia (NH3), we calculate its molar mass as 1 * 14.01 g/mol (for nitrogen) + 3 * 1.01 g/mol (for hydrogen) = 17.04 g/mol. This molar mass signifies the mass of 6.022 x 10^23 molecules of ammonia.
- Define molar mass and explain its significance in chemistry.
Molar Mass: The Foundation of Chemistry
In the enchanting tapestry of chemistry, molar mass stands as an indispensable thread, weaving together the fundamental elements that shape our world. It’s a concept that can initially appear daunting, but with a touch of storytelling, we can unravel its intricacies and unveil its significance.
Imagine a vast library, where countless books line the shelves, each representing a different chemical substance. The molar mass of each book is like a unique identifier, telling us how many pages (molecules) it contains per kilogram. This information is crucial for understanding the composition and properties of the substances we work with.
By unraveling the formula for calculating molar mass, we gain the power to decode these numerical secrets. It’s a simple equation: molar mass = grams per mole * atomic mass. Here, grams per mole represents the mass of one mole of a substance, while atomic mass is the average mass of its atoms.
With the periodic table as our guide, we can navigate the atomic world and uncover the hidden information encoded in each element’s symbol. The periodic table is a map that organizes elements based on their atomic numbers, which tell us the number of protons in their nuclei. This number, combined with the number of neutrons, determines the atomic mass of the element.
Atomic masses are not always whole numbers. This is because most elements exist as a mixture of isotopes, which are atoms of the same element with different neutron numbers. The mass number, which is the sum of protons and neutrons, can vary among isotopes. When calculating atomic mass, we consider the average mass of all isotopes weighted by their relative abundance.
Finally, we encounter the concept of molecular formula, a concise code that reveals the arrangement of atoms within a molecule. The molecular formula of ammonia (NH3), for instance, tells us that each molecule contains one nitrogen atom and three hydrogen atoms. By summing the atomic masses of these atoms, we can determine the molar mass of ammonia and understand its molecular weight.
Understanding molar mass is like holding a key that unlocks the secrets of the chemical world. It allows us to determine the composition, properties, and reactions of substances with precision. So, let us embrace this fundamental concept, for it is the foundation upon which the edifice of chemistry is built.
Formula for Molar Mass: Unveiling the Essence of Chemical Measurement
In the realm of chemistry, understanding the concept of molar mass is paramount. It represents the mass of one mole of a substance, a fundamental unit of measurement in the field. To calculate molar mass, a simple formula comes into play:
Molar Mass = Grams per Mole × Atomic Mass
Grams per Mole: The Bridge between Mass and Quantity
Grams per mole is a crucial constant that converts mass (measured in grams) to amount (measured in moles). It serves as the bridge between the macroscopic and microscopic realms of chemistry, allowing us to determine the number of entities (atoms, molecules, ions) present in a given sample.
Atomic Mass: The Keystone of Molar Mass
Atomic mass represents the average mass of an atom of an element, taking into account its various isotopes. Isotopes are atoms of the same element with different numbers of neutrons, leading to slight variations in mass. The periodic table provides a convenient tool for determining atomic masses, with each element’s row and column providing essential information.
Elements are arranged in the periodic table according to their atomic number, which indicates the number of protons in the nucleus. Atomic mass, on the other hand, is influenced by both the number of protons and neutrons. As we move down a column (group) in the periodic table, atomic mass generally increases due to the addition of more energy levels and electrons. Across a row (period) from left to right, atomic mass also tends to increase as the number of protons and neutrons increases.
Unveiling the Secrets of the Periodic Table: Its Role in Determining Atomic Mass
Embark on a captivating journey through the periodic table, the cornerstone of chemistry, where we unravel its profound role in deciphering the enigmatic atomic mass. The periodic table, an organized arrangement of elements, holds the key to comprehending the intricacies of chemical formulas and their profound significance in unlocking the secrets of matter.
Elements and Atomic Numbers: The Building Blocks of Matter
Within the periodic table, each element occupies a unique position, identified by its atomic number. This pivotal number represents the quantity of protons found within the nucleus of an atom. Protons, along with neutrons, contribute to the mass of an atom, providing us with a foundation for understanding atomic mass.
Periodic Trends: A Journey Through Atomic Mass
As we traverse the periodic table, intriguing trends emerge. Moving across each period, from left to right, atomic number increases, paralleled by a gradual increase in atomic mass. This pattern arises from the addition of protons and neutrons to the nucleus.
Conversely, when descending a group, atomic number increases, but atomic mass exhibits a more nuanced behavior. While atomic mass generally increases, anomalies exist. For instance, in Group 18 (noble gases), atomic mass decreases as we progress down the column. This seemingly paradoxical behavior can be attributed to the inert nature of noble gases, where electron configurations favor stability over the addition of neutrons.
Uniting the Elements: The Molecular Formula
Molecular formulas provide the blueprint for understanding the composition of compounds, the fundamental building blocks of our world. Each element within a compound is represented by its chemical symbol, accompanied by a subscript indicating the number of atoms present. By combining the atomic masses of each constituent element, we can determine the molecular mass of the compound.
For example, let’s unravel the molecular formula of ammonia (NH3). Nitrogen (N) resides in Group 15 with an atomic number of 7, while hydrogen (H) belongs to Group 1 with an atomic number of 1. Plugging these values into the formula for molecular mass:
Molecular Mass = (1 x Atomic Mass of N) + (3 x Atomic Mass of H)
= (1 x 14.01 amu) + (3 x 1.01 amu)
= 17.04 amu
This meticulous formula unveils the molar mass of ammonia, providing essential information for unraveling the mysteries of chemical reactions and their implications in the tapestry of our natural world.
Atomic Mass: The Defining Characteristic of Elements
In the realm of chemistry, understanding the atomic mass of an element is crucial for unraveling its properties and behavior. Atomic mass is the cornerstone of chemistry, providing a glimpse into the very essence of matter.
Imagine an atom as a tiny universe, with a nucleus at its heart, surrounded by swirling electrons. The nucleus harbors protons and neutrons, subatomic particles that determine the atom’s identity and mass. Atomic mass is a measure of the mass of an atom, expressed in atomic mass units (amu).
The atomic mass of an element is not a fixed value. Instead, it reflects the varied nature of its atoms. Atoms of the same element can have different numbers of neutrons, resulting in variations in their masses. These variations are known as isotopes.
Each isotope has a characteristic mass number, which represents the total number of protons and neutrons in its nucleus. The mass number is crucial in calculating the atomic mass. While protons contribute 1 amu each, neutrons contribute slightly less, with each neutron adding approximately 1 amu to the mass.
Calculating Atomic Mass Using Isotopes
To determine the atomic mass of an element, we must consider all its isotopes and their relative abundances. The atomic mass is a weighted average of the masses of its isotopes, taking into account their proportions in nature.
For instance, the element chlorine has two stable isotopes: chlorine-35 (75.78%) and chlorine-37 (24.22%). Chlorine-35 has a mass number of 35, while chlorine-37 has a mass number of 37. Using the following formula, we can calculate the atomic mass of chlorine:
Atomic mass = (mass of isotope 1 x abundance of isotope 1) + (mass of isotope 2 x abundance of isotope 2) + ...
Plugging in the values for chlorine, we get:
Atomic mass of chlorine = (35 amu x 75.78%) + (37 amu x 24.22%) = 35.453 amu
Therefore, the atomic mass of chlorine is 35.453 amu, a reflection of the contributions of its two isotopes. Understanding atomic mass is essential for exploring the periodic trends, chemical reactions, and countless other phenomena that shape our world.
Unveiling the Molecular Formula: Its Significance in Chemistry
Molecular Formula: A Blueprint of Molecular Structure
In the realm of chemistry, understanding the molecular formula is akin to unlocking the secret code that decipher the building blocks of molecules. A molecular formula portrays the exact number and type of atoms that constitute a specific molecule. It serves as a roadmap that reveals the molecular weight and molar mass, two critical parameters for comprehending chemical reactions and stoichiometry.
Molecular Weight vs. Molar Mass
Molecular weight represents the sum of the atomic masses of all the atoms in a molecule. Molar mass, on the other hand, is the mass of one mole of a substance. One mole is defined as the amount of substance that contains exactly 6.022 x 10^23 particles (Avogadro’s number). Therefore, the molar mass of a substance is numerically equal to its molecular weight expressed in grams per mole.
Example: Calculating Molar Mass
Consider ammonia (NH3). Its molecular formula indicates that each molecule consists of one nitrogen atom (N) and three hydrogen atoms (H). Using the periodic table, we find the atomic mass of nitrogen to be 14.01 atomic mass units (amu) and that of hydrogen to be 1.01 amu.
Molar Mass of NH3 = (1 × Atomic Mass of N) + (3 × Atomic Mass of H)
= (1 × 14.01 amu) + (3 × 1.01 amu)
= 14.01 amu + 3.03 amu
= 17.04 amu
Therefore, the molar mass of ammonia is 17.04 grams per mole. This value is essential for determining the mass of a specific quantity of ammonia molecules and for performing calculations involving chemical reactions and stoichiometry.