Understanding Molar Enthalpy: A Comprehensive Guide For Energy-Related Applications
Molar enthalpy, a measure of thermal energy contained within a mole of a substance, provides valuable insights into energy-related phenomena. It can be determined through calorimetry, particularly using bomb calorimeters for combustion reactions. Hess’s Law and enthalpy of formation facilitate indirect enthalpy calculations. Molar enthalpy has significant applications in predicting reaction feasibility, combustion processes, calorimetry, and specific heat capacity measurements, making it a crucial concept in chemistry, physics, and engineering.
Unveiling the Science Behind Molar Enthalpy: A Comprehensive Guide
In the realm of chemistry and thermodynamics, the concept of molar enthalpy plays a crucial role in understanding energy-related phenomena. Enthalpy, a thermodynamic function, measures the total energy of a system, including its thermal energy and the work it can do. Molarity, expressing the concentration of a solution, and concentration, a measure of the amount of solute in a given volume of solution, are equally important parameters in this field.
Molar enthalpy, specifically, provides valuable insights into the energy changes that accompany chemical reactions. It represents the amount of energy contained by one mole of a substance, offering a standardized measure for comparing the energy content of different substances. This makes it particularly significant in predicting the feasibility and efficiency of chemical processes.
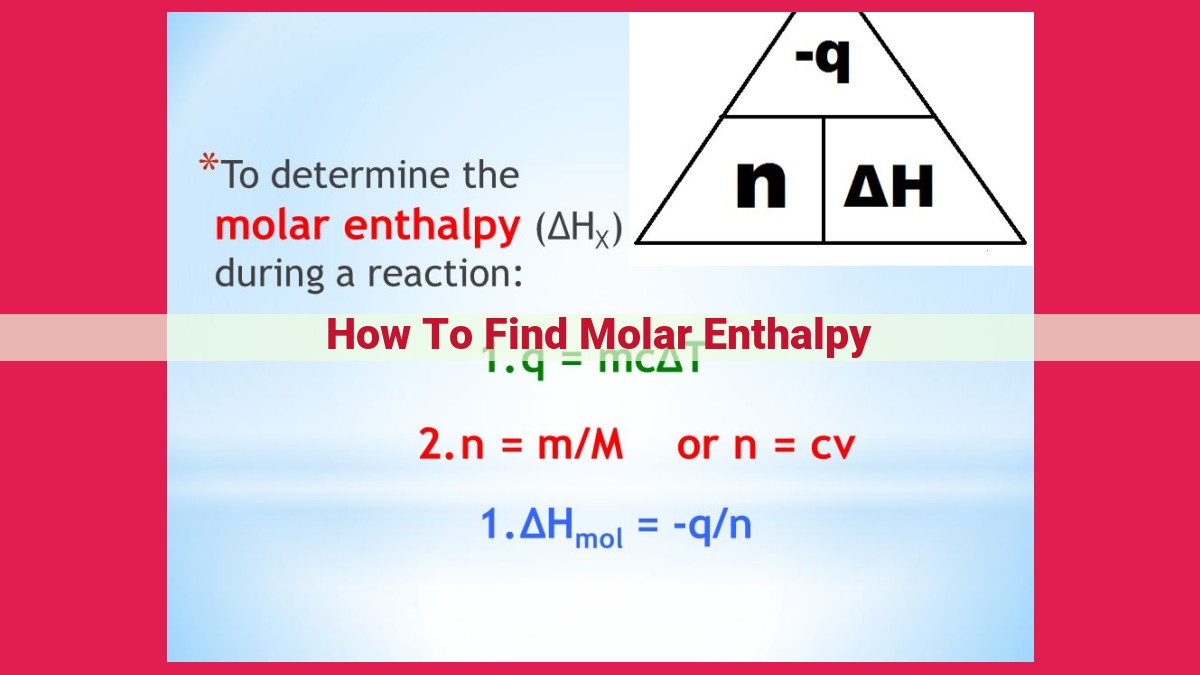
Molar Enthalpy Calculations: Unveiling Energy Changes
Imagine a chemical reaction, a dance of molecules exchanging energy. Enthalpy, like a currency of energy, measures the total energy change in this dance. To quantify this energy exchange, scientists rely on molar enthalpy, a measure of enthalpy change per mole of substance.
To determine molar enthalpy, we employ a key technique called calorimetry. It involves monitoring temperature changes as a reaction occurs. The simplest calorimeter is a coffee cup calorimeter, where the reaction’s heat causes a temperature rise in the solution. However, for highly exothermic or endothermic reactions, a more precise tool is required – the bomb calorimeter.
The bomb calorimeter is a sealed vessel containing a sample and pure oxygen. The reaction occurs inside, generating heat that raises the temperature of the surrounding water. By measuring the temperature change and knowing the mass of water, scientists can calculate the molar enthalpy of the reaction.
Specifically, the bomb calorimeter determines the heat of combustion, a measure of energy released when a substance burns completely. This information is crucial for understanding fuel efficiency and designing engines.
In summary, calorimetry and the bomb calorimeter are indispensable tools for measuring molar enthalpy. These techniques provide valuable insights into the energy dynamics of chemical reactions, with implications for fields like fuel science, chemical engineering, and thermodynamics.
Related Concepts
To understand molar enthalpy, let’s delve into the world of thermal energy, energy, and heat. Thermal energy is the energy associated with the temperature of a substance. Think of it as the energy that makes things hot or cold.
Now, energy is a fundamental concept that refers to the ability to do work. It exists in various forms, such as thermal energy, kinetic energy (energy of motion), and potential energy (stored energy).
Heat is a specific form of energy transfer that occurs when there is a temperature difference between two objects or systems. When heat is transferred, the total energy of the system changes.
The relationship between these concepts and molar enthalpy is crucial. Molar enthalpy captures the energy change associated with a chemical reaction or physical transformation. It can be calculated by measuring the heat flow that occurs during the process. By understanding how thermal energy, energy, and heat are interconnected, we gain a deeper appreciation of molar enthalpy’s significance in various scientific and engineering fields.
Methods for Finding Molar Enthalpy
Unveiling the secrets of molar enthalpy, we venture into the captivating world of thermodynamics. In this captivating journey, we’ll explore the ingenious methods that scientists have devised to uncover its enigmatic nature.
Hess’s Law: A Masterstroke of Indirect Measurement
Like a detective unraveling a complex case, Hess’s Law provides a clever way to indirectly determine enthalpy changes. It unveils a fundamental principle: the total enthalpy change for a reaction is independent of the pathway taken. By skillfully combining multiple known enthalpy changes, we can deduce the enthalpy change for the desired reaction without direct measurement. Isn’t that a stroke of brilliance?
Enthalpy of Formation: A Key to Unlocking Enthalpy
Enthalpy of formation, a pivotal concept in the realm of thermodynamics, emerges as the enthalpy change associated with the formation of one mole of a compound from its constituent elements in their standard states. Armed with this knowledge, we can effortlessly calculate molar enthalpy changes for various reactions by tapping into a treasure trove of tabulated enthalpy of formation values. It’s like having a magical key that unlocks the secrets of chemical transformations.
Applications of Molar Enthalpy: Unlocking the Secrets of Energy
Understanding the concept of molar enthalpy is crucial for comprehending various energy-related phenomena, especially in the fields of chemistry and engineering. This blog post will delve into the practical applications of molar enthalpy and unravel its importance in predicting chemical reactions, analyzing combustion processes, and facilitating calorimetry measurements.
Predicting the Feasibility of Chemical Reactions
Molar enthalpy plays a pivotal role in determining the feasibility of chemical reactions. A chemical reaction is considered exothermic if it releases energy, indicated by a negative molar enthalpy change. Conversely, endothermic reactions absorb energy, resulting in a positive molar enthalpy change.
Understanding molar enthalpy allows scientists and engineers to predict the spontaneity of reactions. Negative molar enthalpy changes suggest that the reaction will occur spontaneously, while positive values indicate that the reaction requires external energy to proceed.
Combustion Processes: Unveiling the Energy of Fuel
Combustion processes, such as burning fuels, are heavily influenced by molar enthalpy. The heat of combustion, which represents the amount of energy released during complete combustion, can be calculated using molar enthalpy. This information is essential for designing efficient combustion systems, such as engines and power plants.
By understanding the molar enthalpy of fuels, scientists can optimize combustion processes to maximize energy output while minimizing environmental impact.
Calorimetry and Specific Heat Capacity: Measuring Thermal Energy
Molar enthalpy is also crucial in calorimetry, the study of heat transfer. Using calorimeters, scientists measure the amount of heat absorbed or released during a reaction. The specific heat capacity, a measure of a substance’s ability to absorb heat, can also be calculated using molar enthalpy.
These measurements are essential in various fields, including material science and biochemistry, for characterizing materials and understanding biological processes.
Molar enthalpy proves to be an indispensable tool for unraveling the secrets of energy-related phenomena. Its applications range from predicting chemical reactions to optimizing combustion processes and facilitating calorimetry measurements. By understanding molar enthalpy, scientists and engineers can harness the power of energy in various fields, leading to advancements in technology and a deeper understanding of our world.