Calculating Millimoles (Mmol): A Guide For Chemistry, Biochemistry, And Nutrition
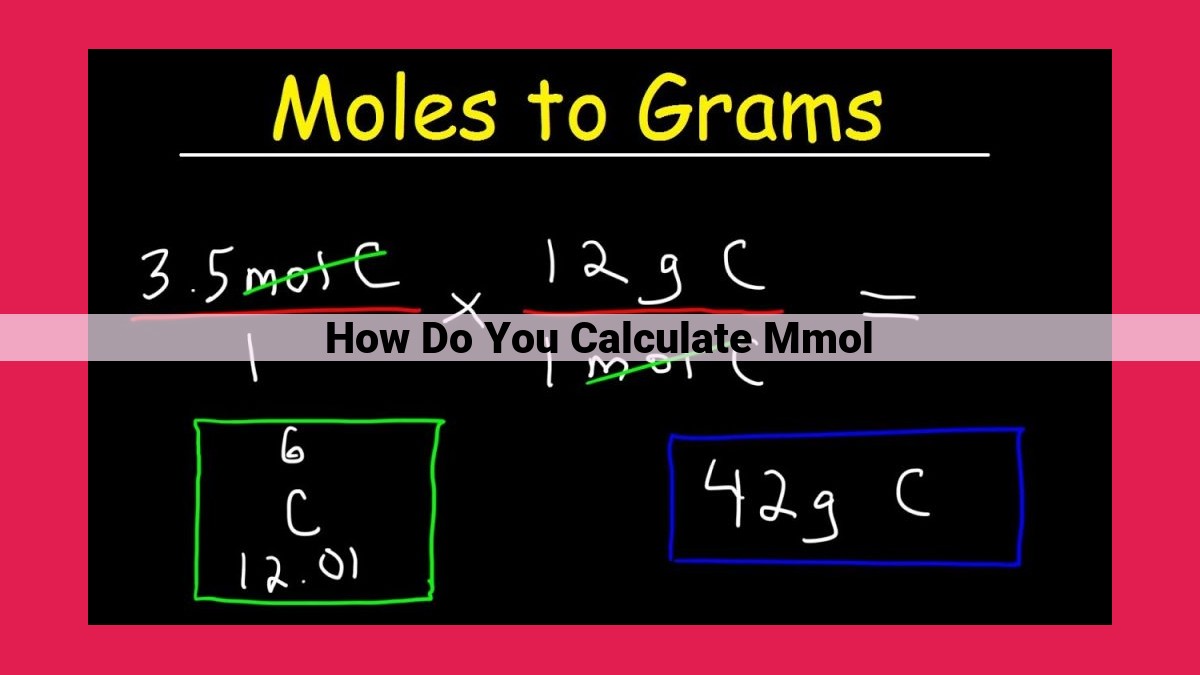
To calculate millimoles (mmol), determine the mass of the substance in grams and divide it by its molar mass in grams per mole. Molar mass is the mass of one mole of a substance and can be found from the periodic table or reference resources. For instance, 100 grams of glucose (C6H12O6) has a molar mass of 180.16 g/mol, so it contains 100 g / 180.16 g/mol = 0.555 mmol of glucose. Millimoles are commonly used in chemistry, biochemistry, and nutrition to measure the amount of a substance, determine concentrations, and perform stoichiometric calculations.
Definition of Millimole
- Explain what a millimole (mmol) is and its significance in measuring the amount of a substance.
- Compare it to the mole (mol) as the standard unit for quantifying substance amount.
Understanding Millimoles: A Beginner’s Guide
In the world of science, understanding the amount of a substance is crucial for various calculations and experiments. While the mole is the standard unit for measuring substance amount, the millimole (mmol) offers a convenient way to quantify smaller quantities. Understanding millimoles is essential for students, researchers, and professionals in various fields.
A millimole is a thousandth of a mole (1 mmol = 0.001 mol). It’s a unit that represents the amount of a substance containing exactly 6.02214076 × 10^23 constituent particles. This number is known as Avogadro’s number.
The mole is the preferred unit for quantifying the amount of a substance, particularly in chemistry. However, the millimole is often more appropriate when dealing with smaller quantities. For instance, in biochemistry and nutrition, millimoles are commonly used to express concentrations of various substances, such as glucose.
Understanding Molar Mass: The Key to Converting Grams to Moles
In the realm of chemistry, molar mass plays a pivotal role in bridging the gap between the macroscopic world of grams and the microscopic world of moles. Understanding this concept is essential for grasping the quantitative nature of chemical substances.
Molar mass embodies the mass, expressed in grams, of one mole of a substance. It’s a fundamental property of each element or compound, revealing the mass of its constituent atoms or molecules. For instance, the molar mass of water (H2O) is 18.015 grams per mole.
To uncover the molar mass of a substance, we embark on an adventure filled with periodic table exploration. Each element graces the periodic table with its atomic mass, the average mass of its naturally occurring isotopes. By examining their position and value, we can swiftly determine the molar mass of the element.
Alternatively, we can seek refuge in reference resources, such as the CRC Handbook of Chemistry and Physics, which provide a wealth of molar mass data. With these resources at our fingertips, we can effortlessly retrieve molar masses for more complex compounds, like glucose, whose molar mass stands at 180.15 grams per mole.
Your journey to master molar mass continues as we delve into the practical applications of this concept. In the realm of chemistry, biochemistry, and nutrition, millimoles, micromoles, and other units derived from molar mass reign supreme. Armed with this knowledge, you’ll conquer stoichiometry, determine concentrations, and unravel various chemical mysteries.
Calculating Millimoles: A Simple Guide
Understanding millimoles is essential for various scientific fields. To calculate millimoles, we use a straightforward formula:
**mmol = Mass (g) / Molar Mass (g/mol)**
Let’s break down each part of this formula:
- Mass (g): This refers to the mass of the substance you’re interested in, measured in grams.
- Molar Mass (g/mol): This is the mass per mole of the substance, a fundamental property of each element or compound. It tells us how many grams of the substance are present in one mole of it.
Determining Mass and Molar Mass
To calculate millimoles accurately, it’s crucial to obtain accurate values for both mass and molar mass.
Mass (g): You can typically measure the mass of a substance using a balance or scale.
Molar Mass (g/mol): You can find the molar mass of a substance in several ways:
- Periodic Table: For elements, you can find their molar masses directly on the periodic table.
- Reference Resource: For compounds, you can consult a reference book or online database to find their molar masses.
Example: Glucose Calculation
Let’s calculate the millimoles of glucose present in 10 grams of glucose. The molar mass of glucose is 180.153 g/mol.
**mmol of Glucose = Mass (g) / Molar Mass (g/mol)**
**mmol of Glucose = 10 g / 180.153 g/mol**
**mmol of Glucose = 0.0555 mmol**
Therefore, 10 grams of glucose contain 0.0555 mmol of glucose.
Millimoles: A Deeper Understanding for Everyday Science
In the world of science, understanding the amount of a substance is crucial for various calculations and applications. One key concept is the millimole (mmol), which plays a significant role in quantifying substances.
Understanding Millimoles and Molar Mass
The millimole represents a specific quantity of a substance, similar to the mole (mol), the standard unit for measuring substance amount. While a mole contains 6.022 × 10^23 particles (known as Avogadro’s number), a millimole represents one-thousandth (1/1000) of that amount.
To convert between mass and moles (or millimoles), we use molar mass, which represents the mass of one mole of a substance. We can find molar mass from the periodic table or reference resources.
Calculating Millimoles with Glucose
Let’s take glucose as an example. The molar mass of glucose is 180.153 g/mol. This means that 180.153 grams of glucose is equivalent to 1 mole of glucose.
To calculate the number of millimoles in a given mass of glucose, we use the formula:
Millimoles (mmol) = Mass (g) / Molar Mass (g/mol)
Suppose we have 5 grams of glucose. To find the equivalent number of millimoles:
Millimoles (mmol) = 5 g / 180.153 g/mol = 0.0277 mmol
Therefore, 5 grams of glucose is equivalent to 0.0277 millimoles of glucose.
Applications of Millimoles
Millimoles are widely used in various fields, including:
- Chemistry: Determining concentrations and stoichiometry in chemical reactions.
- Biochemistry: Calculating concentrations of molecules in biological systems.
- Nutrition: Expressing the content of vitamins, minerals, and other nutrients in foods.
Other Related Units
Besides millimoles, there are other related units like micromoles (µmol), which represent one-millionth (1/1,000,000) of a mole. Conversion factors can help us move between these units as needed.
Understanding millimoles is essential for accurately measuring and quantifying substances in science. By grasping the concepts of molar mass and performing millimole calculations, we can unlock a deeper understanding of various scientific fields.
Additional Resources:
Applications of Millimole
- Describe how millimoles are used in various fields, such as chemistry, biochemistry, and nutrition.
- Discuss their importance in determining concentrations, stoichiometry, and other calculations.
Applications of Millimoles
Millimoles, symbolized as mmol, play a crucial role in various scientific disciplines, particularly in chemistry, biochemistry, and nutrition. Their significance stems from their utility in determining the amount of a substance, facilitating precise measurements and calculations.
In chemistry, millimoles are essential for determining the concentration of solutions. Solution concentration, expressed in molarity, represents the number of moles of solute per liter of solution (mol/L). By converting the mass of the solute to millimoles (using its molar mass), we can easily determine the molar concentration. This knowledge is vital for stoichiometric calculations, which involve determining the reactants and products in chemical reactions in precise ratios.
Millimoles are also widely used in biochemistry, where they aid in quantifying the concentration of biological molecules, such as enzymes, proteins, and nucleic acids. Understanding the millimolar concentration of these molecules is crucial for studying enzyme kinetics, metabolic pathways, and genetic mechanisms.
In the field of nutrition, millimoles are employed to express the dietary intake of essential nutrients. These include minerals like calcium, potassium, and magnesium, as well as vitamins such as vitamin C and vitamin B12. Millimoles provide a standardized unit of measurement for comparing nutrient intake across different individuals and population groups, enabling better assessment of nutritional status and dietary requirements.
Millimole vs. Micromole and Other Units
- Introduce micromole (µmol) and other units related to millimoles.
- Explain the conversion factors between different units.
Millimole vs. Micromole and Other Units
Understanding millimoles is crucial in quantifying substances, but there are other related units that you may encounter:
-
Micromole (µmol): A micromole is one-thousandth of a millimole: 1 µmol = 0.001 mmol. It’s commonly used in biochemistry for measuring small amounts of substances.
-
Mole (mol): The mole is the standard unit for measuring substance amount, and it contains a specific number of entities called Avogadro’s number (approximately 6.022 × 10^23). 1 mol = 1000 mmol.
-
Kilomole (kmol): A kilomole is equal to 1000 moles: 1 kmol = 1000 mmol. It’s used for quantifying large amounts of substances.
Conversion Factors
To convert between these units, use the following factors:
- 1 mol = 1000 mmol
- 1 mmol = 1000 µmol
- 1 kmol = 1000 mmol
Practical Example
Let’s say you have 5 grams of glucose (C6H12O6). Its molar mass is 180.153 g/mol. To convert grams to millimoles, use the formula:
mmol = Mass (g) / Molar Mass (g/mol)
mmol = 5 g / 180.153 g/mol
mmol = 27.76 mmol
Now, let’s convert this value to micromole:
µmol = mmol x 1000
µmol = 27.76 mmol x 1000
µmol = 27,760 µmol
Therefore, 5 grams of glucose is equivalent to 27.76 millimoles or 27,760 micromoles.
Additional Resources
- Provide links to reference materials, calculators, or other helpful resources for understanding millimoles and their applications.
Understanding Millimoles: A Comprehensive Guide
In the realm of chemistry and science, the concept of millimoles (mmol) plays a crucial role in measuring the amount of a substance. It’s a derived unit of the mole (mol), the standard unit for quantifying the amount of a substance.
Molar Mass: The Key to Conversion
To understand millimoles, we need to delve into the concept of molar mass. This is the mass of one mole of a substance, expressed in grams per mole (g/mol). Simply put, it’s the mass of all the atoms or molecules in one mole of that substance.
Calculating Millimoles: A Formula
With molar mass firmly grasped, we can now calculate millimoles using the formula:
mmol = Mass (g) / Molar Mass (g/mol)
To determine the mass in grams and the molar mass in grams per mole, refer to a periodic table or a reliable reference resource.
Practical Example: Glucose and Millimoles
Let’s illustrate this calculation with a specific substance: glucose. Glucose is a sugar molecule with a molar mass of 180.156 g/mol. Suppose we have 100 grams of glucose. To convert this to millimoles, we simply plug these numbers into the formula:
mmol = 100 g / 180.156 g/mol = 0.555 mmol
Applications of Millimoles
Millimoles find myriad applications across fields such as chemistry, biochemistry, and nutrition. They’re used to determine concentrations, perform stoichiometric calculations, and quantify the amount of a substance in various contexts.
Millimole Equivalents: Micromoles and More
The concept of millimoles is closely intertwined with other units, such as micromoles (µmol). Micromoles are 1/1000 of a millimole, providing a more precise unit for very small amounts of substances.
Additional Resources for Your Curiosity
For further exploration, here are some helpful resources:
By grasping the concept of millimoles and their applications, you’ll gain a deeper understanding of the quantitative aspects of chemistry and other scientific disciplines.