Understanding Mixtures: Types, Properties, And Their Significance
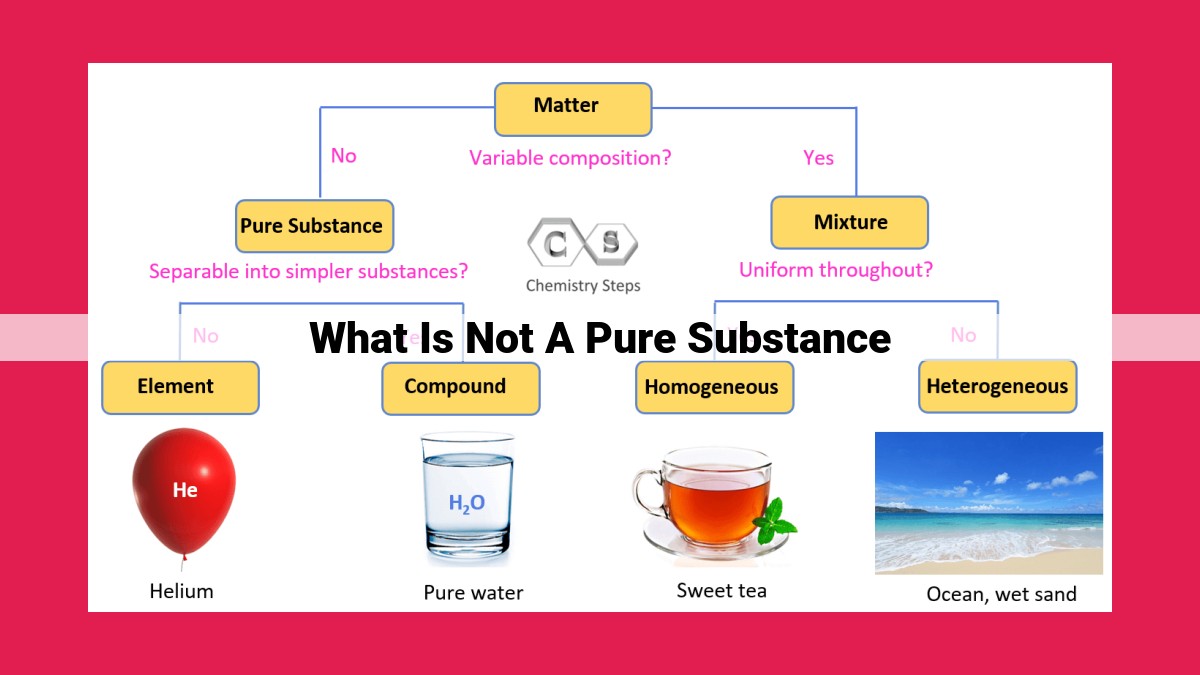
Anything that is not a pure substance is a mixture. Mixtures are combinations of two or more chemical substances that are not chemically bonded. The components of a mixture retain their identity and are mixed in different forms, such as solutions, colloids, or suspensions. In heterogeneous mixtures, the components are unevenly distributed, forming distinct phases. In homogeneous mixtures, the components are evenly distributed, forming a single phase. Solutions are homogeneous mixtures where the solute is dissolved in the solvent. Colloids are heterogeneous mixtures where the particles are larger than those in solutions but smaller than those in suspensions. Suspensions are heterogeneous mixtures where the particles are large enough to settle out over time.
Defining Pure Substances
- Explain what constitutes a pure substance, including its elemental composition and uniform properties.
Defining Pure Substances: The Essence of Uniformity
In the fascinating realm of chemistry, we encounter an intriguing distinction between pure substances and mixtures. Pure substances, the building blocks of matter, possess an inherent simplicity and homogeneity that set them apart. They are composed of only one type of element or a specific combination of elements, forming a distinct chemical entity.
Imagine a pristine lake, its azure waters shimmering beneath the summer sun. This body of water represents a pure substance. Every molecule within it is identical, composed entirely of hydrogen and oxygen in a fixed ratio. The lake’s uniform properties, such as temperature and density, are a testament to its purity.
In contrast to pure substances, mixtures are assemblages of two or more different substances that retain their own identities. They can be further classified into heterogeneous and homogeneous mixtures, depending on the distribution of their components.
Heterogeneous Mixtures: A Patchwork of Distinct Ingredients
When substances come together, they can form different types of mixtures. One such type is a heterogeneous mixture, where the components are unevenly distributed, creating distinct phases that are easily observable.
Imagine a bowl filled with granola, a crunchy mix of oats, nuts, and dried fruits. Each ingredient has its own unique characteristics and is clearly visible within the mixture. The oats form a base, while the nuts and fruits are scattered throughout, creating separate phases. This uneven distribution is a hallmark of heterogeneous mixtures.
Another example is a salad. The crisp lettuce, juicy tomatoes, crunchy croutons, and savory cheese are all distinct components that can be easily identified. When tossed together, they create a colorful and heterogeneous mixture. The dressing, acting as a solvent, evenly coats the ingredients but does not dissolve them, preserving their individual identities.
These examples illustrate the key feature of heterogeneous mixtures: the coexistence of distinct phases within the same concoction. This lack of uniformity gives heterogeneous mixtures their unique properties and makes them easily distinguishable from other types of mixtures.
Homogeneous Mixtures: A Tale of Uniformity
In the captivating world of chemistry, we encounter substances with diverse compositions and properties. Amidst this variety, homogeneous mixtures stand out as a class of substances that enchant us with their remarkable uniformity.
Defining Homogeneous Mixtures
Homogeneous mixtures, the harmony of nature, are substances composed of two or more components that are so intimately intertwined that they appear to the naked eye as a single, indistinguishable entity. Unlike their heterogeneous counterparts, homogeneous mixtures present a uniform appearance, as if the components have seamlessly blended together.
Examples of Homogeneous Mixtures
Everyday life abounds with examples of homogeneous mixtures that grace our routines. Air, the lifeblood of our planet, is a prime illustration of a homogeneous mixture, its composition remaining constant regardless of location. Similarly, salt water, the vast expanse of our oceans, is a homogeneous mixture of salt and water, its salinity consistent throughout.
Uniform Distribution of Components
The hallmark of homogeneous mixtures lies in the uniform distribution of their components. This harmonious arrangement results in a single phase, where no distinct boundaries exist between the components. In contrast, heterogeneous mixtures exhibit multiple phases, where the components are clearly discernible.
Mechanisms of Homogeneity
The achievement of homogeneity in mixtures often relies on processes like diffusion and dissolution. Diffusion, the spontaneous movement of particles from an area of higher concentration to an area of lower concentration, ensures the thorough mixing of components. Dissolution, the process of solute particles dispersing into solvent particles, further contributes to the formation of homogeneous mixtures.
Importance of Homogeneous Mixtures
Homogeneous mixtures play a pivotal role in various scientific and industrial applications. They form the basis of many chemical reactions, providing a uniform environment for reactants to interact. Moreover, their consistent composition makes them essential for analytical chemistry, where precise measurements are crucial.
In conclusion, homogeneous mixtures captivate us with their seamless unity, revealing the profound effects of uniform distribution of components. From the life-sustaining air we breathe to the vast oceans that envelop our planet, homogeneous mixtures epitomize the harmonious blending of nature’s elements. Their unique properties and wide-ranging applications underscore their significance in both the scientific realm and everyday life.
Solutions: Solutes, Solvents, and Concentrations
- Define solutions and introduce the concepts of solute, solvent, and concentration.
- Provide real-world examples of solutions.
Solutions: A Blend of Solutes and Solvents
Imagine yourself at a colorful seaside, where the azure ocean meets the sandy shore. This captivating scene represents a natural solution: a uniform blend of salts and minerals dissolved in the vast expanse of water. In chemistry, we encounter similar scenarios involving solutions, solutes, and solvents.
A solution is a homogeneous mixture where the components are evenly distributed throughout, forming a single, indistinguishable phase. This harmonious union arises due to the intimate interaction between two types of substances:
-
Solute: The guest substance that dissolves or disperses into another substance. It is typically present in a smaller quantity.
-
Solvent: The host substance that dissolves or disperses the solute. It is usually present in a larger quantity.
The concentration of a solution, a crucial parameter in chemistry, describes the relative amounts of solute and solvent. It can be expressed in various units, such as molarity (moles of solute per liter of solution), mass percent (grams of solute per 100 grams of solution), or volume percent (milliliters of solute per 100 milliliters of solution).
In our everyday lives, we encounter countless examples of solutions. The salty sea is a solution of various salts dissolved in water. Alcoholic beverages are solutions of ethanol dissolved in water. Even our bodies are home to intricate solutions, such as blood (red blood cells suspended in plasma) and urine (waste products dissolved in water). Understanding the nature of solutions is essential in various scientific disciplines, including medicine, environmental science, and material science.
Colloids: Tyndall Effect and Brownian Motion
- Define colloids and explain the Tyndall effect and Brownian motion.
- Discuss examples of colloids, highlighting their unique properties.
Colloids: The Enigmatic World of Tiny Particles
In the realm of chemistry, not all mixtures are created equal. Suspensions, solutions, and colloids occupy unique spaces, each with distinct characteristics. Today, we delve into the fascinating world of colloids, exploring their perplexing nature and the remarkable phenomena that define them.
Colloids: An Intermediate Realm
Imagine a world where tiny particles, too small to be visible to the naked eye but larger than molecules, dance in suspension. These particles, called colloidal particles, form colloids, a class of mixtures that bridge the gap between heterogeneous mixtures (with visible components) and homogeneous mixtures (with evenly distributed components).
The Tyndall Effect: Shining a Light on Colloids
When a beam of light passes through a colloid, something extraordinary occurs. The light scatters in all directions, illuminating the path of the light beam like a celestial roadmap. This phenomenon, known as the Tyndall effect, is a telltale sign of the presence of colloidal particles. The scattering occurs because the colloidal particles are large enough to interact with the light waves, causing them to deflect and scatter.
Brownian Motion: The Dance of Tiny Particles
Peer into the microscopic world of a colloid, and you’ll witness a captivating ballet. Colloidal particles, suspended in a fluid, exhibit a ceaseless, random motion known as Brownian motion. Named after the botanist Robert Brown, who first observed this phenomenon in 1827, Brownian motion is caused by the incessant bombardment of the particles by countless molecules in the surrounding fluid.
The constant bombardment imparts a seemingly erratic motion to the colloidal particles, causing them to zigzag and twirl in a perpetual dance. This dance provides valuable insights into the behavior of molecules and particles at the nanoscale.
Examples of Colloids: From Milk to Fog
Colloids are not confined to the laboratory; they are ubiquitous in nature and everyday life. Some notable examples include:
- Milk: A white, opaque liquid, milk is a colloid of fat globules suspended in water. The light scattering by these globules gives milk its characteristic color.
- Fog: A hazy curtain that envelops the landscape, fog is a colloid of tiny water droplets suspended in air. The Tyndall effect scatters light, reducing visibility and creating the ethereal glow of fog.
- Paint: The smooth, even finish of paint is achieved through the colloidal suspension of pigment particles in a liquid medium. The colloidal nature prevents the pigment particles from settling or clumping, ensuring a uniform paint application.
In conclusion, colloids are a mesmerizing class of mixtures that occupy a unique space between heterogeneous and homogeneous mixtures. The Tyndall effect and Brownian motion reveal the intricate world of colloidal particles, providing insights into the behavior of matter at the nanoscale. From the milky white of milk to the ethereal glow of fog, colloids are a testament to the hidden wonders that lie all around us.
Suspensions: Witnessing the Gravity-Defying Dance of Particles
Have you ever shaken a bottle of orange juice or stirred up a glass of muddy water? If so, you’ve created a suspension, a fascinating mixture where tiny particles defy gravity and remain suspended within a liquid. In this realm of the suspension, we’ll explore the captivating world of sedimentation and settling time.
Unveiling Suspensions: A Tale of Two Phases
Suspensions are heterogeneous mixtures, meaning they consist of two distinct phases: a dispersed phase and a dispersion medium. The dispersed phase comprises solid particles or liquid droplets that are suspended within the dispersion medium, which is typically a liquid.
Sedimentation: The Gradual Descent of Particles
Over time, the suspended particles in a suspension begin to settle due to gravity. This process is known as sedimentation. As the particles descend, they collide with each other and with the dispersion medium, slowing their descent.
Settling Time: Measuring the Pace of Sedimentation
The settling time of a suspension refers to the amount of time it takes for a certain percentage of the suspended particles to settle to the bottom of the container. This time is influenced by various factors, including the size and density of the particles, the viscosity of the dispersion medium, and the presence of any stabilizing agents.
Examples of Suspensions: From Mud to Blood
Suspensions are found in countless everyday substances, including:
- Muddy water: Suspended sediment particles give rivers and lakes their murky appearance.
- Blood: Red blood cells are suspended within a liquid plasma.
- Paint: Pigment particles are suspended in a liquid binder.
- Milk: Butterfat globules are suspended in a liquid serum.
Suspensions are a testament to the intricate interactions between gravity and particles within a liquid medium. From muddy rivers to the life-sustaining blood flowing through our veins, suspensions play a vital role in our world. Understanding the processes of sedimentation and settling time provides a glimpse into this captivating realm of chemistry and physics.