Mitochondrial Respiration: Unveiling The Energy Conversion Process
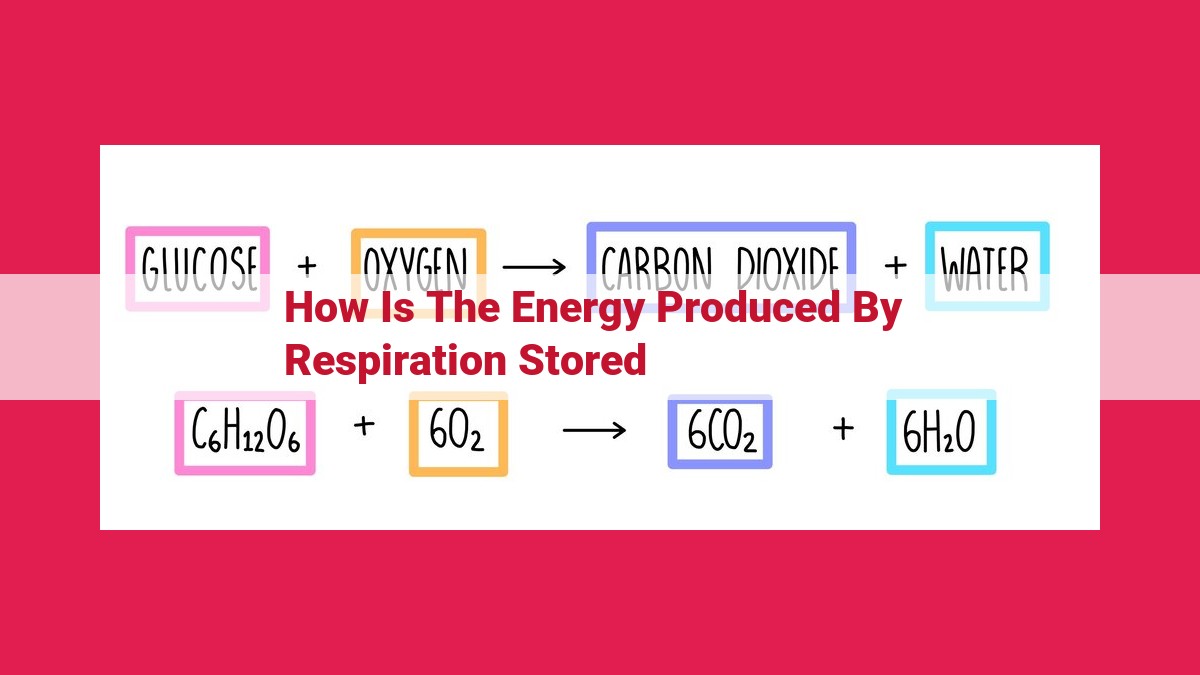
During respiration, the electron transport chain generates energy through redox reactions, releasing energy that pumps protons across a membrane. This proton gradient drives chemiosmosis, where protons flow through ATP synthase, causing its rotation and promoting ATP synthesis from ADP. The energy initially stored in glucose is thus converted into a usable form of energy, ATP.
Electron Transport Chain: The Powerhouse of the Cell
Imagine your body as a bustling metropolis, teeming with countless tiny factories working tirelessly to sustain life. These factories, known as cells, rely on a steady supply of energy to power their intricate operations. At the heart of this energy production process lies a crucial machinery called the electron transport chain.
The electron transport chain is a complex network of proteins embedded within the mitochondrial membrane. Its primary function is to harness the energy released during the transfer of electrons, a process known as redox reactions. As electrons pass through the chain like a baton in a relay race, they lose energy, which is captured and stored.
Each step in the electron transport chain involves the reduction (gain of electrons) of one molecule and the oxidation (loss of electrons) of another. This energy-releasing cascade ultimately culminates in the reduction of oxygen, the final electron acceptor.
ATP Production: Harnessing Energy for Cellular Processes
Our cells are bustling powerhouses, constantly producing energy to fuel life’s processes. At the heart of this energetic machinery lies the electron transport chain, a series of protein complexes that act as tiny power generators within our cells.
As electrons pass through these complexes, they lose energy, which is then used to pump protons across a membrane. These protons don’t just sit there; they create a proton gradient, a difference in concentration between the two sides of the membrane. It’s like a battery, storing energy in the form of protons.
This proton gradient is the key to ATP production. ATP (adenosine triphosphate) is the energy currency of our cells. It’s like a rechargeable battery that cells use to power everything from muscle contractions to protein synthesis.
To generate ATP, cells rely on a molecular machine called ATP synthase. This enzyme has a rotating head that, when powered by the proton gradient, drives the phosphorylation of ADP (adenosine diphosphate) to ATP. It’s like a water wheel that uses the flow of protons to generate energy.
So, there you have it, the intricate dance of electron transfer, proton pumping, and ATP synthesis. It’s a symphony of molecules that keeps our cells energized and our bodies humming.
Oxidative Phosphorylation: Unleashing the Potential of the Proton Gradient
The electron transport chain, like an energy-generating powerhouse, sets the stage for a series of intricate processes that culminate in the creation of ATP, the body’s primary energy currency. Through a remarkable mechanism known as oxidative phosphorylation, the proton gradient established across the mitochondrial membrane is harnessed, unlocking the potential for ATP production.
This process, reminiscent of a molecular symphony, begins with the movement of protons across the membrane. The energy released from the electron transport chain pumps protons into the intermembrane space, creating a disparity in proton concentration. This gradient, like a miniature battery, stores immense energy.
Enter ATP synthase, a microscopic masterpiece, poised to exploit this proton gradient. As protons flow back down the gradient, through a channel within ATP synthase, a remarkable phenomenon occurs. The protein complex rotates, its intricate machinery resembling a cellular turbine. This rotation drives the enzyme’s catalytic action, enabling the synthesis of ATP from ADP.
The rotation of ATP synthase, like a spinning wheel, facilitates a chemical transformation. ADP, the raw material, enters the enzyme’s active site, where a phosphate group is attached, transforming it into ATP, the energy-rich molecule. This process, repeated countless times, generates the ATP that fuels cellular activities, powering the body’s intricate processes.
ATP Synthase: The Molecular Powerplant Fueling Cellular Processes
In the realm of cellular energy production, a remarkable molecular machine known as ATP synthase plays a pivotal role. This intricate structure harnesses the power of a proton gradient, acting as a microscopic powerplant that drives the synthesis of ATP, the universal energy currency of cells.
Nestled within the inner mitochondrial membrane, ATP synthase is a complex protein that spans across the membrane. Its role is to convert the energy stored within the proton gradient into a form that cells can readily utilize. The proton gradient, established by the electron transport chain, represents a reservoir of energy that ATP synthase taps into.
As protons flow down the gradient, they pass through ATP synthase’s central stalk, causing it to rotate. This rotation triggers a series of conformational changes within ATP synthase, leading to the phosphorylation of ADP into ATP. The rotation of the stalk is coupled to the active site of ATP synthase, where ADP binds and is converted into ATP.
The process of ATP synthesis, known as oxidative phosphorylation, is an essential step in cellular respiration. It is through oxidative phosphorylation that the energy stored in glucose is converted into ATP, providing the fuel for a multitude of cellular processes, including muscle contraction, nerve impulse transmission, and protein synthesis.
In essence, ATP synthase is the molecular powerplant of cells. It harnesses the energy of the proton gradient to generate ATP, the energy currency that powers cellular life. Its intricate structure and precise movements are a testament to the elegance and efficiency of nature’s design.
Proton Gradient: The Driving Force Behind Cellular Energy
Cellular respiration is the process by which cells generate the energy necessary to power their activities. This intricate process involves a series of chemical reactions that ultimately convert the energy stored in glucose into ATP (adenosine triphosphate), the universal energy currency of cells. At the heart of cellular respiration lies a process known as oxidative phosphorylation, which utilizes a proton gradient to generate ATP.
The proton gradient is established by the electron transport chain, a series of proteins embedded in the inner membrane of mitochondria. As electrons pass through this chain, they undergo a series of redox reactions, releasing energy. This energy is used to pump protons from the mitochondrial matrix into the intermembrane space, creating a concentration gradient.
The proton gradient is a crucial component of oxidative phosphorylation. It drives the process of chemiosmosis, whereby protons flow back into the mitochondrial matrix through a specific protein complex called ATP synthase. As protons pass through ATP synthase, they cause its central stalk to rotate. This rotation, in turn, triggers a conformational change that facilitates the phosphorylation of ADP (adenosine diphosphate) into ATP.
The proton gradient is thus fundamental to the generation of ATP, the energy molecule that powers cellular processes. Without it, oxidative phosphorylation and ATP synthesis would not be possible, and cells would lack the energy to carry out their essential functions. The proton gradient is a testament to the extraordinary efficiency of cellular respiration, allowing cells to harness the energy stored in glucose and convert it into the chemical energy required for life.