Metallic Bonding: A Deep Dive Into The “Sea” Of Delocalized Electrons
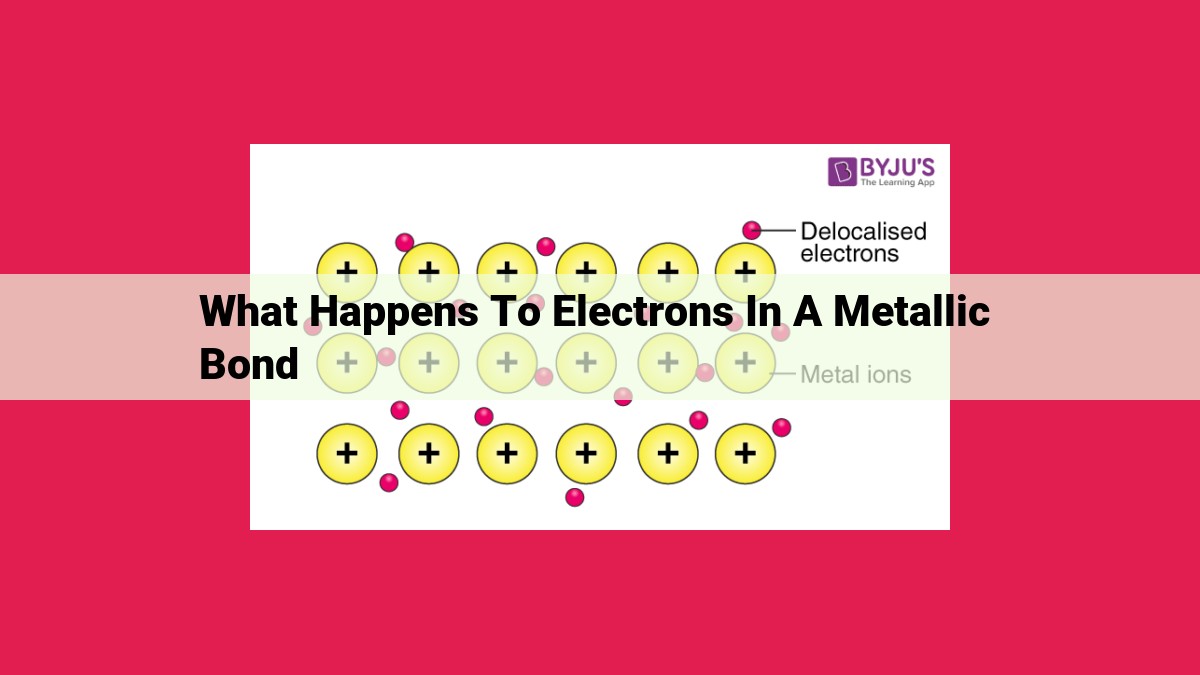
In metallic bonds, valence electrons are loosely bound to metal atoms, creating a “sea” of free electrons. These free electrons interact with positively charged metal ions, resulting in the delocalization of valence electrons. This allows electrons to move freely throughout the metal, facilitating electrical and thermal conductivity. This delocalization of electrons explains the unique properties of metals, such as their ability to reflect light and their malleability.
The Extraordinary World of Metals: Unveiling the Secrets of Their Unique Properties
Metals stand out in the realm of materials, captivating us with their distinct qualities. Their strength, malleability, and remarkable ability to conduct electricity and heat have forged their indispensable role in our technological advancements. But what lies beneath these remarkable properties? It all boils down to the intricate dance of electrons.
Metallic Bonds: The Keystone of Metal’s Character
The distinctive properties of metals stem from the exceptional behavior of electrons within their metallic bonds. In these bonds, valence electrons—the outermost electrons of metal atoms—break free from their atomic confines. This liberation creates a sea of free electrons that seamlessly flows throughout the entire metal structure.
This sea of free electrons is the key player in determining metals’ remarkable qualities. Freed from the clutches of individual atoms, these electrons are highly mobile, allowing metals to conduct electricity with exceptional ease. They also contribute to metals’ malleability and ductility, allowing them to be shaped and molded without breaking.
Delving into the Electron Sea Model
The electron sea model vividly portrays the dynamic world within metals. Imagine a vast ocean of electrons, surging and swirling around positively charged metal ions. These ions, stripped of their valence electrons, reside at fixed positions in the metal’s crystal lattice. The free electrons, liberated from their atomic prisons, dance freely through this electron sea, constantly interacting with the ions.
Valence Electrons: The Unsung Heroes
Valence electrons are the unsung heroes of metallic bond formation. These outermost electrons are loosely bound to their parent atoms, making them easily removable. This readiness to break free is the driving force behind the formation of the free electron sea. The number of valence electrons directly influences a metal’s properties, such as its conductivity and reactivity.
Conductivity: The Essence of Metal’s Power
Metals’ conductivity—their ability to transmit electricity—is a direct consequence of their free electron sea. When an electric field is applied to a metal, these free electrons are effortlessly accelerated, carrying an electric current throughout the material. This remarkable property makes metals essential components in electrical systems, from power lines to computer chips.
The Electron Sea: A Fluid Foundation for Metallic Wonders
Welcome to the captivating world of metals, where the unique symphony of electron behavior gives rise to an extraordinary array of properties. We embark on a journey to unravel the secrets behind the electron sea model, the cornerstone of our understanding of metals’ remarkable nature.
Imagine a metal lattice as a vast ocean of positive ions, where the valence electrons, the outermost electrons of each metal atom, float freely like a boundless sea. These free electrons are not tethered to any particular atom; instead, they roam the metal’s interior, creating a delocalized electron cloud that envelops the entire lattice.
The electron sea model provides a captivating explanation for the remarkable properties of metals. The delocalized valence electrons facilitate the flow of electricity through metals, enabling them to act as brilliant conductors. When an electric field is applied, the free electrons can move effortlessly, carrying the electrical charge throughout the metal.
Furthermore, the electron sea contributes to the exceptional thermal conductivity of metals. The delocalized electrons can rapidly transfer heat energy, allowing metals to conduct heat with remarkable efficiency. This property makes metals invaluable in applications such as heat sinks and cookware.
In essence, the electron sea model reveals the dynamic nature of metals, where free electrons dance freely, orchestrating their material properties. This sea of electrons forms a continuous medium that imbues metals with their characteristic luster, malleability, and remarkable ability to conduct electricity and heat.
The Metallic Bond: A Tale of Loose Electrons and Positive Ions
Metals possess unique properties that set them apart from other materials, and the key to understanding these properties lies in the remarkable behavior of electrons within metallic bonds.
Imagine a bustling city where people (valence electrons) roam freely like a “sea”. These valence electrons are loosely connected to their metal atoms, giving them an exceptional degree of mobility. As the metal atoms lose their valence electrons, they transform into positively charged ions (metal ions).
The interaction between these positively charged metal ions and the “sea” of free electrons forms the cornerstone of the metallic bond. The free electrons, untethered to any particular atom, can move freely throughout the metal, creating a delocalized electron cloud. This cloud of electrons acts as a glue, holding the positively charged metal ions together and forming a cohesive metallic structure.
The delocalization of valence electrons is crucial for the extraordinary properties of metals. These free electrons are responsible for the exceptional electrical and thermal conductivity that metals exhibit. They allow electricity to flow effortlessly through metals, making them invaluable for wires and electrical components. Additionally, the free electrons facilitate efficient heat transfer, which is why metals are widely used in cookware and heat sinks.
The metallic bond is a captivating dance between free electrons and positively charged metal ions, resulting in the unique properties that make metals indispensable in countless applications.
Valence Electrons: The Key Players in Metal Magic
In the enchanting world of metals, valence electrons play a pivotal role, like the protagonists in a captivating story. These tiny particles, residing on the outermost energy level of metal atoms, hold the secret to the remarkable properties that make metals so treasured.
Valence electrons are like tireless actors, ever ready to detach themselves from their atomic homes. This exceptional mobility allows them to roam freely, forming a collective entity known as the electron sea. Immersed in this boundless ocean of free electrons, metal ions – the positively charged remnants of metal atoms – float gracefully, like islands in a vast electronic sea.
This dynamic interaction between metal ions and free electrons forms the metallic bond, the foundation upon which the unique characteristics of metals rest. The delocalization of valence electrons – their ability to move effortlessly throughout the entire metallic structure – empowers metals with their unparalleled electrical and thermal conductivity, making them indispensable in various applications.
Free Electrons: The Conductive Force of Metals
In the realm of materials, metals stand out for their exceptional properties, which stem from the unique behavior of their electrons. These charged particles play a pivotal role in shaping the very essence of metallic substances.
At the heart of this phenomenon lies the electron sea model. This model envisions a vast expanse of valence electrons, loosely bound to their atoms, forming a “sea” of free electrons. These electrons are not confined to individual atoms but are free to roam throughout the metal structure.
This sea of free electrons serves as the foundation for the metallic bond, the force that holds metal atoms together. The positive charges of the metal ions, formed by the loss of valence electrons, interact with the negatively charged free electrons, creating a dynamic equilibrium. This interaction allows for the delocalization of valence electrons, meaning they are no longer confined to specific atoms.
The delocalization of valence electrons has profound implications for the properties of metals. These free electrons act as a conductive fluid, allowing metals to effortlessly conduct electricity and heat. When an electric current is applied to a metal, the free electrons move freely, carrying the electrical charge throughout the material. Similarly, heat transfer in metals is facilitated by the movement of these electrons, carrying thermal energy from one part of the metal to another.
The presence of free electrons bestows upon metals their malleability and ductility, giving them the ability to be shaped and molded without breaking. This is because the free electrons can move freely between atoms, allowing the metal to deform without compromising its integrity.
In summary, free electrons are the key players in the extraordinary properties of metals. Their delocalization allows for the formation of the metallic bond, which in turn facilitates conductivity, malleability, and ductility. These properties make metals indispensable in a wide range of applications, from electrical wiring and thermal conductors to structural components and decorative elements.
**The Marvel of Metals: Unraveling the Secrets of Their Conductivity**
In the realm of materials, metals stand out as remarkable conductors, facilitating the effortless flow of electricity and heat. This extraordinary ability is rooted in their unique electronic configurations and the intricate interplay of forces that govern their metallic bonds.
At the heart of this phenomenon lies the electron sea model, a captivating concept that envisions valence electrons as a boundless “sea” of freely roaming particles within the metal. These electrons, loosely bound to their respective ions, detach themselves with remarkable ease, leaving behind a mesmerizing realm of ethereal, delocalized electrons.
The metallic bond itself is a mesmerizing dance between metal ions and these free electrons. The positively charged ions, anchored within a lattice structure, attract the negatively charged electrons, forming an unbreakable bond. This mesmerizing union allows for the unrestricted movement of valence electrons, giving rise to the remarkable electrical and thermal conductivity that defines metals.
These free electrons, like tireless travelers, meander effortlessly through the metallic lattice, creating a pathway for electricity to dance through. This uninterrupted flow of electrons enables metals to excel as conductors, carrying electrical currents with unparalleled efficiency.
Furthermore, the same free electrons also serve as proficient heat carriers, shuttling thermal energy with exceptional ease. Heat, the relentless agitation of molecules, is effectively dissipated by these nimble electrons, granting metals their signature ability to conduct heat with remarkable speed.
In conclusion, the remarkable conductivity of metals is a testament to the intricate interplay of electron behavior and metallic bonding. The electron sea model, with its boundless sea of free electrons, and the metallic bond, with its unwavering dance between ions and electrons, create a symphony of conductivity that underpins the technological marvels that shape our modern world.