Mercury: Atomic Number 80 And Its Significance In Element Identification
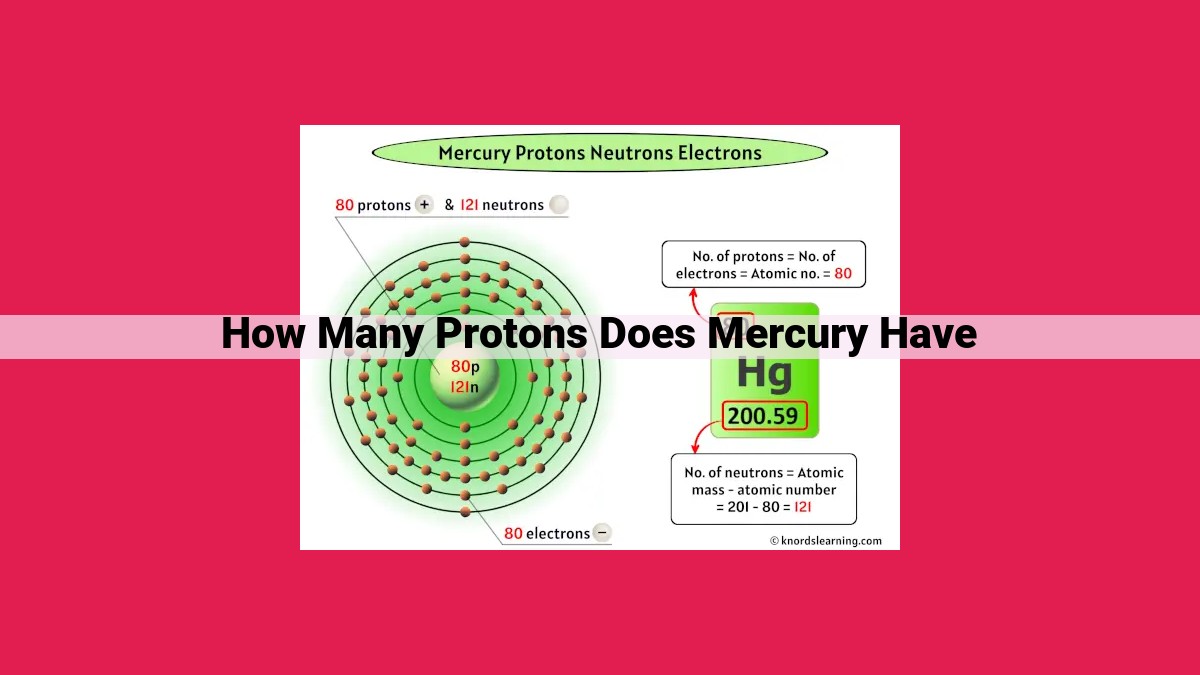
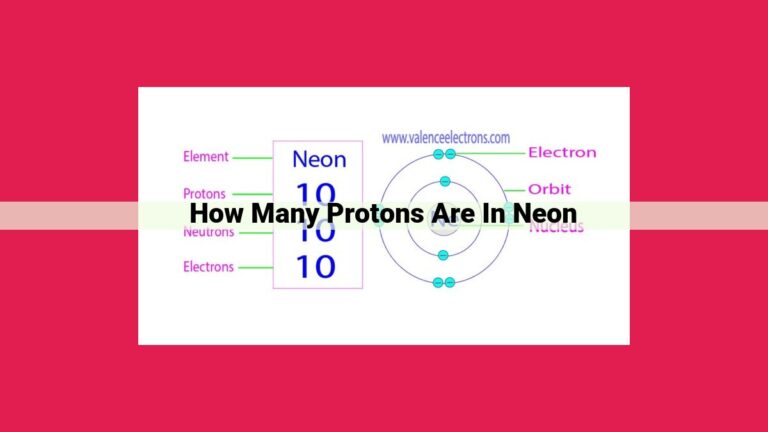
Mercury’s atomic number is 80, indicating it has 80 protons in its nucleus. The atomic number, which is the number of protons, uniquely defines an element and determines its position on the periodic table. Protons reside in the atom’s nucleus along with neutrons, while electrons orbit the nucleus. The combination of protons and neutrons in the nucleus determines the atomic number, which influences an element’s chemical properties and behaviors. Mercury’s atomic number of 80 places it as the 80th element on the periodic table, shaping its distinct chemical characteristics.
Decoding the Essence of an Element: The Atomic Number
In the realm of chemistry, every element unveils its unique identity through a fundamental numerical descriptor: the atomic number. This enigmatic number holds the key to understanding an element’s essence and its place in the tapestry of the periodic table. Join us as we delve into the fascinating world of atomic numbers, using the enigmatic element mercury as our guide.
Atomic Number: The Element’s Fingerprint
Picture this: the heart of an atom, the nucleus, where protons and neutrons reside. The number of protons within this nucleus, like a unique fingerprint, defines the element. Each element possesses a distinct atomic number, a number that remains constant, immutably defining its identity. This number forms the basis of the periodic table, organizing elements in a logical progression based on their atomic numbers.
Mercury’s Atomic Signature: 80
In the case of mercury, a silvery-white liquid metal, its atomic number is 80. This means that every mercury atom harbors 80 protons within its nucleus. It’s this unique atomic number that distinguishes mercury from all other elements. In the periodic table, mercury occupies its rightful place in Group 12 (formerly known as IIB), sharing a home with other elements bearing similar chemical characteristics.
A Symphony of Subatomic Particles
To fully grasp the atomic number’s significance, we must venture into the realm of subatomic particles. Within the atom’s nucleus, protons, with their positive charge, reside alongside neutrons, devoid of any electrical charge. These nuclear partners, along with the electrons orbiting the nucleus, form the fundamental building blocks of matter.
The Nucleus and the Atomic Number
The atomic number, as we’ve established, stems from the number of protons within the nucleus. Every element’s atomic number is unique, and it’s the net number of protons (after accounting for any electrons lost or gained) that bestows an element with its distinctive chemical properties.
Mercury’s Chemical Character
The atomic number of 80 profoundly influences mercury’s chemical behavior. For instance, mercury’s ability to form compounds with other elements, its reactivity, and its physical properties are all shaped by its atomic number. Mercury’s unique chemical fingerprint makes it a versatile element with applications in a wide array of fields, including medicine, chemistry, and industry.
In conclusion, the atomic number is a fundamental property that defines an element’s identity and position on the periodic table. Understanding atomic numbers empowers us to unravel the intricate tapestry of chemistry, enabling us to comprehend the unique properties and behaviors of elements like mercury.
Unveiling the Secrets of Mercury: Exploring Its Atomic Number 80
As we delve into the realm of chemistry, we encounter a fundamental concept that underpins the very nature of elements: the atomic number. This unique identifier serves as a defining characteristic for each element, revealing its elemental identity and shaping its chemical destiny.
Mercury’s Atomic Number: A Tale of 80
Mercury, a name synonymous with liquid silver, possesses an atomic number of 80. This number signifies that every mercury atom harbors 80 protons nestled within its nucleus, the heart of the atom. These protons, along with neutrons, form the core of the atom, while electrons orbit the nucleus, creating an intricate dance of subatomic particles.
Protons, Electrons, and Neutrons: The Subatomic Ensemble
Atoms, the fundamental building blocks of matter, are composed of three subatomic particles: protons, electrons, and neutrons. Protons, positively charged, reside in the nucleus, while electrons, their negatively charged counterparts, whirl around the nucleus in defined energy levels. Neutrons, as their name suggests, carry no electrical charge and also reside in the nucleus.
The Nucleus and Atomic Number
The nucleus, the atom’s dense core, houses both protons and neutrons. The collective number of these subatomic particles determines the atomic number of an element. In mercury’s case, its atomic number of 80 indicates that it possesses 80 protons and a specific number of neutrons, giving it its distinct atomic identity.
Mercury’s Unique Position on the Periodic Table
The atomic number of an element dictates its position on the periodic table, an organizational chart of elements arranged based on their atomic numbers and chemical properties. Mercury’s atomic number of 80 places it in Group 12 and Period 6 of the periodic table, alongside other transition metals with similar chemical characteristics.
Subatomic Particles: The Building Blocks of Atomic Identity
At the heart of every atom lies a bustling community of subatomic particles, each playing a crucial role in defining the unique characteristics of the element. Protons, with their positive charge, reside in the atom’s nucleus, forming its central core. Electrons, on the other hand, are the nimble dancers of the atom, orbiting around the nucleus like planets. These negatively charged particles balance out the positive charge of the protons, maintaining the atom’s electrical neutrality.
But there’s another player in this subatomic symphony: neutrons. These neutral particles, also found in the nucleus, act as harmonizers, balancing the forces between protons. While they don’t carry an electrical charge, their presence adds to the atom’s mass, providing stability and preventing the protons from repelling each other.
The combination of protons and neutrons in the nucleus determines an element’s atomic number. This unique number identifies each element on the periodic table and plays a pivotal role in dictating its chemical properties and behavior.
**The Nucleus: The Heart of the Atom and the Key to Atomic Identity**
At the very core of every atom lies a bustling metropolis of subatomic particles, the nucleus. This tiny, dense center is home to two fundamental particles: protons and neutrons. Protons, armed with positive electric charges, reside within the nucleus, while neutrons, lacking any electrical charge, provide stability and cushion the protons from their repulsive forces.
The combined number of protons and neutrons in the nucleus governs an atom’s atomic number. This number is a veritable fingerprint for each element, uniquely identifying it among the vast tapestry of nature’s building blocks. The atomic number determines not only the element’s position on the periodic table but also its chemical behavior.
In the case of mercury, with its atomic number of 80, the nucleus buzzes with 80 protons, establishing its undeniable identity as the 80th element in the chemical family. These 80 protons, together with a complement of neutrons, form the nucleus of every mercury atom, providing the foundation for its unique properties.
Mercury’s Unique Identity on the Periodic Table
Atomic Number: The Guiding Star
In the tapestry of elements, each thread is identified by its unique atomic number, a code embedded in the heart of its atoms. For mercury, this number is 80, a beacon that defines its position and properties on the periodic table.
The Atomic Orchestra
An atom is a symphony of subatomic particles, a nucleus teeming with protons and neutrons, surrounded by a harmonious chorus of electrons. Protons, with their positive charge, determine an element’s atomic number. Neutrons, neutral in nature, add mass and stability to the nucleus.
The Nucleus and Atomic Identity
The nucleus, the atom’s central core, houses protons and neutrons. Their combined number dictates the atomic number, which is a defining characteristic of each element. It’s like a cosmic fingerprint, unique to each element, assigning it a specific place on the periodic table.
Mercury’s Niche on the Periodic Table
Mercury’s atomic number of 80 places it in the transition metal group, nestled between gold and thallium. This location reflects its shared chemical characteristics, including its silvery appearance, high density, and malleability.
Chemical Dance and Atomic Number
The atomic number not only determines an element’s position on the periodic table but also influences its chemical behavior. Elements with similar atomic numbers tend to exhibit similar chemical properties. This understanding allows scientists to predict and explore the reactivity of different elements.
Mercury’s atomic number of 80 is its unique identifier, defining its position on the periodic table and shaping its chemical properties. It’s a testament to the power of atomic structure in classifying and understanding the elements that make up our world.
How Atomic Number Shapes an Element’s Chemical Character
Every element in the vast tapestry of our universe is defined by its unique atomic number. This numerical fingerprint, like a celestial address, allows us to identify and distinguish one element from another. Mercury, with its atomic number of 80, stands out as a shimmering silver liquid with a captivating story to tell.
An atom’s nucleus, its heart and soul, is where we find the protons and neutrons. These subatomic particles, like cosmic dancers, determine the element’s atomic number – the number of protons that reside within that tiny core. Mercury’s 80 protons grant it the atomic number 80, making it the 80th element on the periodic table’s grand stage.
The atomic number, like a conductor’s baton, orchestrates the element’s symphony of chemical behaviors. It governs the number of electrons orbiting the nucleus, the invisible force that governs chemical bonding. Mercury’s chemical properties, shaped by its 80 electrons, bestow upon it a curious duality. It can dance with other elements as a liquid metal or vanish into the air as a toxic vapor.
Imagine mercury as a cunning alchemist, transforming its character based on its partnerships. With oxygen, it forms a vibrant red oxide, a testament to its ability to form covalent bonds. In the presence of halogens, it dances a more subdued waltz, creating ionic compounds with subtle colors. Mercury’s chemical versatility is a testament to the profound influence of its atomic number, the beacon that defines its chemical destiny.
In the tapestry of the periodic table, mercury’s atomic number of 80 grants it a unique position. Its heavy atomic nucleus and compact electron cloud contribute to its high density and low volatility. Mercury’s unique properties, shaped by its atomic number, make it a fascinating subject of study, a testament to the profound impact of this fundamental number on an element’s chemical character.