Maximize Chromatographic Separation With High-Performance Liquid Chromatography (Hplc)
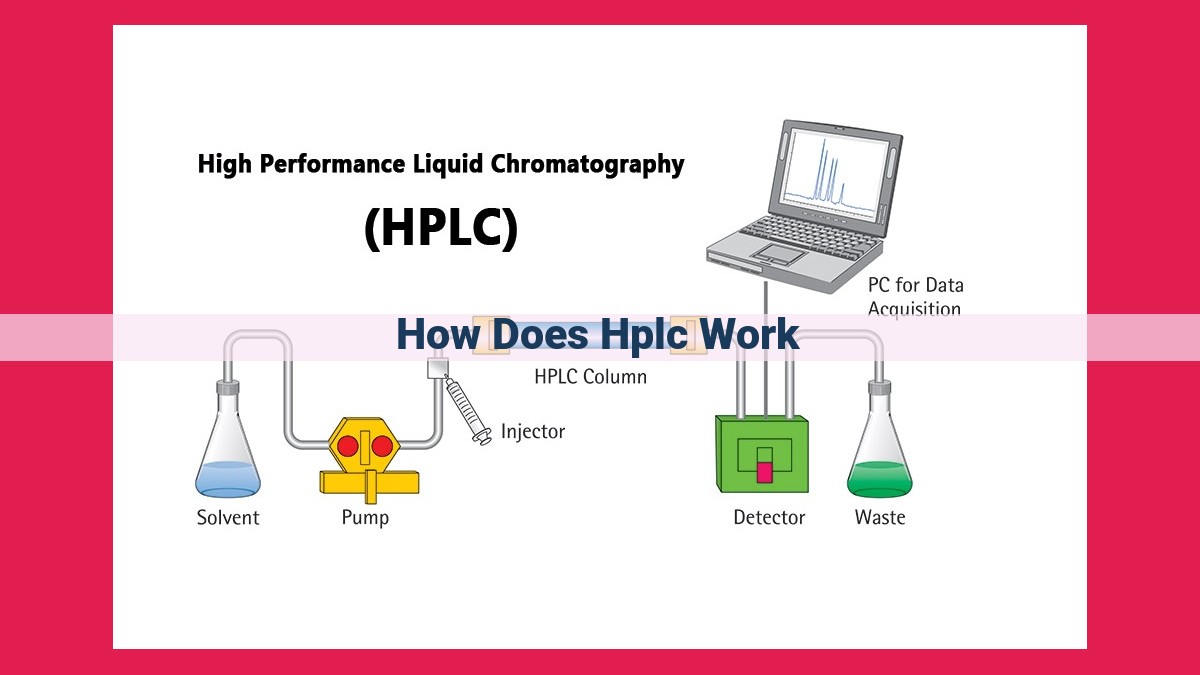
HPLC employs column chromatography, where a liquid sample is passed through a stationary phase packed within a column. Components interact with the stationary phase based on their chemical properties, leading to varying retention times. A mobile phase transports the sample through the column, separating components as they elute. Different mobile and stationary phase combinations optimize separation, while detectors like UV-Vis or fluorescence detect separated components. The resulting chromatogram displays separated components as peaks, with retention time used for identification and quantification. HPLC’s versatility makes it widely applicable in fields like pharmaceutical, environmental, and food analysis, aiding in compound identification, purification, and quality control.
Unlocking the Power of HPLC: A Guide to Separation and Purification
As scientists embark on the quest for understanding and manipulating the intricate world of molecules, they encounter a myriad of challenges. High-performance liquid chromatography (HPLC) emerges as an indispensable tool in this pursuit, offering an elegant and versatile solution for separating and purifying complex mixtures.
Imagine yourself as an intrepid explorer venturing into a dense forest teeming with an unfathomable number of plants. Each plant, with its unique characteristics and properties, holds secrets waiting to be uncovered. To unravel these mysteries, you need a way to disentangle the intricate web of life that nature has woven.
HPLC is akin to a molecular scalpel, providing the ability to dissect and separate these components with unrivaled precision. It harnesses the power of liquid chromatography, a technique that separates molecules based on their interactions with a stationary phase, typically packed into a column.
This seemingly simple concept belies the remarkable versatility of HPLC. By carefully selecting the stationary and mobile phases, scientists can tailor the separation to the specific properties of their target molecules. Imagine a tailored suit, custom-made to fit the unique shape and size of each molecular species.
The journey through the HPLC column is a dynamic dance between the analytes (the molecules being separated) and the stationary phase. Those with a stronger affinity for the stationary phase will linger longer during their passage, while those with a weaker affinity will glide through more swiftly.
By analyzing the retention time of each component, scientists can unravel their identity and even quantify their abundance. The resulting chromatogram becomes a visual map, a treasure trove of molecular secrets laid bare.
HPLC has become an invaluable tool in a vast array of scientific disciplines. From the development of new drugs to ensuring food safety, from environmental monitoring to understanding the mysteries of biology, HPLC empowers researchers and industry professionals alike.
So, as you embark on your scientific adventures, remember the power of HPLC, the molecular scalpel that empowers you to unlock the secrets of the molecular world.
HPLC: Unraveling the Secrets of Separation and Purification
In the realm of scientific analysis, High-Performance Liquid Chromatography (HPLC) stands tall as a powerful separation and purification technique. Imagine it as a meticulous sorter, meticulously dividing complex mixtures into their individual components. HPLC’s principles are founded upon the differential affinities of substances towards a stationary phase within a column. As a liquid mobile phase carrying the sample flows through the column, the components interact with the stationary phase, resulting in their separation.
Column Chromatography: The Bedrock of HPLC
HPLC’s foundation lies in the concept of column chromatography. Just as a prospector sifts through soil to extract gold, HPLC employs a column packed with a stationary phase that selectively retains specific components of the sample. The stationary phase can be tailored to bind to specific molecules, allowing the desired components to be captured and purified.
Mobile and Stationary Phases: The Dynamic Duo
In the dance of separation, the mobile and stationary phases play complementary roles. The mobile phase is a solvent or mixture of solvents that transports the sample through the column. Its composition, flow rate, and temperature determine how components interact with the stationary phase. The stationary phase, on the other hand, is a solid or liquid coating within the column that provides the surface for selective interactions with the sample components.
To achieve the most effective separation, a gradient elution technique is often employed. Here, the composition or strength of the mobile phase is gradually changed over time. This dynamic approach allows the separation of complex mixtures by gradually altering the affinities of components towards the stationary phase.
Column Chromatography: The Foundation of HPLC
Imagine you’re a chemist tasked with separating a complex mixture of chemicals into its individual components. Enter column chromatography, the cornerstone of High-Performance Liquid Chromatography (HPLC).
Picture this: A long, narrow glass column filled with a solid material, known as the stationary phase, acts as the battleground for your separation. The liquid mixture, or mobile phase, carrying your compounds flows through this column from top to bottom.
As the mobile phase traverses the stationary phase, different components of your mixture begin to interact. Some components cling more tightly to the stationary phase, slowing their progress, while others glide through with ease. This differential interaction is the key to separating your mixture.
Compounds with a stronger affinity for the stationary phase will spend more time in the column, resulting in a longer retention time. On the other hand, compounds with a weaker affinity will pass through the column more quickly, resulting in a shorter retention time.
By carefully selecting the stationary phase and mobile phase, you can tailor the separation to your specific needs. This delicate balance between stationary and mobile phases is what empowers HPLC to separate complex mixtures into their individual components.
Column Chromatography: The Foundation of HPLC
HPLC (High-Performance Liquid Chromatography) is a powerful separation and purification technique that revolutionized the field of analytical chemistry. At its core, HPLC relies on the principles of column chromatography.
A column is a tube filled with a porous stationary phase, typically a solid or gel-like material. As a liquid _mobile phase_ flows through the column, a mixture of components passes through this stationary phase. Different substances interact with the stationary phase in varying degrees, **causing them to separate based on their:
- Chemical structure
- Molecular weight
- Polarity
Molecules with stronger interactions with the stationary phase will move more slowly through the column. Conversely, those with weaker interactions will move more quickly. This differential migration leads to the separation of the components in the mixture.
Imagine a race where runners have different shoe treads: some with grippy spikes, others with smooth soles. As they traverse a sandy track, runners with spiked shoes will sink in and move slowly, while those with smooth soles will glide across the surface, outpacing their spiked counterparts. In the same way, molecules with strong interactions with the stationary phase, like Velcro to a fuzzy surface, will lag behind those with weaker interactions, which zip through like a greased pig.
The Dynamic Duo of HPLC: Unlocking the Secrets of Mobile and Stationary Phases
In the realm of scientific analysis, High-Performance Liquid Chromatography (HPLC) stands as a formidable separation and analysis technique, empowering scientists to unravel the complexities of chemical mixtures. At the heart of HPLC’s prowess lies a harmonious interplay between two key players: the mobile phase and the stationary phase.
Picture the mobile phase as a tireless courier, carrying the sample mixture through the intricate labyrinth of the chromatographic column. Its composition, typically a liquid solvent or a mixture thereof, is carefully optimized to ensure proper dissolution and transport of the sample components. As the mobile phase flows through the stationary phase, a solid matrix packed within the column, the components in the sample undergo a selective dance of interactions.
The stationary phase acts as a discerning gatekeeper, selectively interacting with the different sample components. These interactions, known as partitioning, are influenced by the physical and chemical properties of both the components and the stationary phase. Components with a stronger affinity for the stationary phase spend more time interacting with it, ultimately slowing their movement through the column.
The mobile phase then sweeps the separated components along, carrying them further through the column. Those that interact more weakly with the stationary phase are carried through more quickly, while those with a stronger attraction spend more time lagging behind. By carefully controlling the composition and flow rate of the mobile phase, scientists can fine-tune the separation process, ensuring that each component elutes (comes out of the column) at a distinct time.
This intricate interplay between the mobile phase and the stationary phase underpins the remarkable versatility of HPLC. By altering their properties, scientists can tailor the technique to suit a wide range of sample types and applications. From pharmaceutical analysis to environmental monitoring and food safety testing, the dynamic duo of HPLC’s mobile and stationary phases unravels the secrets of complex mixtures, empowering us to understand and manipulate the molecular world around us.
Column Chromatography: The Foundation of HPLC
Column chromatography is the fundamental principle behind HPLC. It involves passing a mixture of substances through a column packed with a stationary phase. As the mixture flows through the column, different components interact with the stationary phase to varying degrees. This interaction is based on specific properties, such as size, charge, and polarity.
Based on these interactions, the components separate as they travel through the column. Components that interact more strongly with the stationary phase will move more slowly, while those that interact less strongly will move faster. This differential movement leads to the separation of the components in the mixture.
The Role of Mobile and Stationary Phases: Key Components of HPLC
The mobile phase in HPLC is a solvent that flows through the column. It acts as a carrier for the sample mixture and helps to elute the components from the column. The stationary phase is a solid or liquid material that is packed into the column. It provides the interactive surface for the components of the mixture to bind to.
The composition and flow rate of the mobile phase significantly impact the separation process. Different mobile phase compositions can change the strength of the interactions between the components and the stationary phase. This can affect the selectivity and efficiency of the separation.
Gradient Elution
A gradient elution technique involves gradually changing the composition of the mobile phase during the separation process. This approach can improve the resolution and separation of complex mixtures. The gradient elution can be programmed to optimize the separation conditions for each component.
Gradient Elution: Unlocking the Secrets of Complex Mixture Separations
In the world of analytical chemistry, when faced with intricate mixtures that pose challenges to traditional separation methods, scientists turn to a technique that wields the power of controlled flow and selective interactions: gradient elution.
Imagine a labyrinth of intricate tunnels, each lined with a different material. As you navigate through this maze, some molecules cling more tightly to certain walls than others. Gradient elution mimics this concept, using a mobile phase that gradually changes its composition as it flows through the column. By carefully manipulating the mobile phase’s properties, scientists can create a dynamic environment that favors the separation of even the most stubborn mixture components.
Initially, the mobile phase is composed of a solvent that weakly interacts with the stationary phase. As the separation progresses, a stronger solvent or modifier is gradually introduced into the mix. This change in composition creates a gradient of strength, causing molecules to elute at different points along the column. Molecules with a stronger affinity for the stationary phase will remain bound longer, while those with a weaker affinity will be swept away by the increasing solvent strength.
The result is a chromatogram that resembles a roadmap of separated components, each represented by a distinct peak. By carefully controlling the gradient, scientists can optimize the separation and identify even trace amounts of target compounds within the complex matrix. Gradient elution has become an invaluable tool, particularly in industries such as pharmaceuticals, environmental analysis, and food science, where intricate mixtures are commonplace. It has empowered chemists to unlock the secrets of these complex concoctions, providing insights into their composition and aiding in scientific breakthroughs and quality control measures.
HPLC Detectors: Unveiling the Secrets of Separated Molecules
In the realm of HPLC (High-Performance Liquid Chromatography), detectors play a pivotal role in unmasking the secrets of separated molecules. These sophisticated devices monitor the elution of components as they pass through the column, providing a visual representation of their presence.
Imagine a stage where a play is unfolding. The performers, our sample components, take their turns crossing the stage. As they do, our detectors, like watchful observers, sense their every move. Based on the specific properties that the detectors are sensitive to, they can identify and quantify these components with remarkable precision.
Detectors in HPLC come in various forms, each with its unique strengths:
UV-Vis Detector: This detector shines ultraviolet or visible light through the eluent, observing how the light interacts with sample components. Those that absorb specific wavelengths reveal their presence, allowing for the detection of compounds with chromophores.
Fluorescence Detector: Similar to the UV-Vis detector, this one relies on fluorescence. Sample components that fluoresce under excitation light emit their own distinctive wavelengths, making their identification even more precise.
Refractive Index Detector: This detector measures the change in refractive index as sample components elute. As the refractive index of a sample is closely related to its concentration, this detector provides direct information about the quantity of each component.
Electrochemical Detector: Employing electrochemical reactions, this detector is highly sensitive to electrochemically active compounds. It is commonly used in the analysis of neurotransmitters, pharmaceuticals, and antioxidants.
Each detector has its own detection principle and sensitivity, making the choice of detector crucial. By understanding the strengths and limitations of these detectors, scientists can harness their power to reveal the hidden intricacies of their samples.
HPLC: Unraveling the Secrets of Sample Detection
In the realm of scientific analysis, High-Performance Liquid Chromatography (HPLC) stands as an indispensable tool, its ability to separate and identify sample components unmatched. One crucial element in this process lies in the detectors employed, each a silent sentinel, sensitively tuned to capture the unique characteristics of substances as they make their journey through the HPLC system.
Imagine an army of detectors, each a specialized soldier with a distinct mission. Ultraviolet detectors, armed with their keen eyesight, absorb light at specific wavelengths, revealing the presence of molecules with light-absorbing structures. Fluorescence detectors become luminous beacons, their emission of light signaling the presence of fluorescent substances.
For substances that elude the scrutiny of light, refractive index detectors step into the fray. These astute sensors detect changes in the refractive index of the solvent, allowing them to identify compounds based on their molecular size and shape. Conductivity detectors, on the other hand, monitor the electrical conductivity of the eluent, providing insights into the presence of ions.
The detectors’ sensitivity to specific properties makes them indispensable for a vast array of applications. In the pharmaceutical industry, HPLC coupled with UV detectors empowers scientists to ensure the purity and potency of drug substances, while fluorescence detectors help unravel the mysteries of complex biological samples. Refractive index detectors play a crucial role in food analysis, detecting sugars, fats, and other components with high precision. And in environmental monitoring, conductivity detectors aid in uncovering the presence of heavy metals and other contaminants.
As we witness the relentless march of HPLC technology, novel detectors continue to emerge, each pushing the boundaries of sensitivity and selectivity. These advancements promise to further expand the scope of HPLC applications, making it an indispensable tool for scientific discovery and technological breakthroughs.
Describe the chromatogram and how it represents separated components.
The Chromatogram: A Story of Separation
In the realm of HPLC, the chromatogram unfolds like a captivating tale, revealing the secrets of your sample’s composition. This intricate tapestry paints a vibrant picture of separated components, each dancing to its own unique melody.
The chromatogram’s graceful curves and peaks represent the telltale signs of your sample’s inhabitants. Each component, as it emerges from the HPLC column, interacts with a detector, sending a signal that paints a peak on the chromatogram’s canvas. The distance along the chromatogram, known as the retention time, serves as a fingerprint, identifying each component.
Just as the ebb and flow of a river’s current influences its inhabitants, the composition and flow rate of the mobile and stationary phases dance together to orchestrate the separation. Components that waltz through the column slowly nestle snugly against the stationary phase, creating larger peaks. Those that glide more swiftly pass by the stationary phase with a quick nod, resulting in smaller peaks.
The chromatogram becomes a visual symphony, offering insights into the complex dance of your sample components. By decoding the story it tells, you gain invaluable knowledge about the chemical composition, purity, and concentration of your sample.
Retention Time: The Key to Identifying and Quantifying Sample Components in HPLC
In the world of HPLC, retention time plays a crucial role in unraveling the identity and abundance of substances within a sample. Imagine you’re a detective investigating a complex crime scene, and retention time is your trusty magnifying glass, helping you piece together evidence.
Retention time represents the time it takes for a specific component in your sample to travel through the HPLC column. It’s like a unique fingerprint for each substance, allowing you to identify them with precision. As the mixture of components flows through the column, each one interacts with the stationary phase differently. Those that interact more strongly will retain longer on the column, while those that interact less will elute (come out of the column) sooner.
This variation in retention times allows you to separate the components based on their specific properties. It’s like a detective using different techniques to isolate suspects based on their unique characteristics. By measuring the time it takes for each component to emerge from the column, you can create a “chromatogram,” a graphical representation of the separation.
The chromatogram is your roadmap to understanding the sample. Each peak on the graph represents a different component, and by calculating its retention time, you can identify it by comparing it to known standards. It’s like matching fingerprints to identify suspects.
Retention time is also essential for quantifying sample components. The peak area in the chromatogram is directly proportional to the amount of that component in the sample. By measuring the peak areas, you can determine the concentration of each component with accuracy.
In essence, retention time is a powerful tool in the hands of HPLC detectives. It allows them to identify and quantify sample components with precision, solving complex analytical puzzles and unlocking a wealth of information about the sample.
HPLC: A Powerful Technique for Separation and Analysis
High-performance liquid chromatography (HPLC) has emerged as an invaluable tool in the scientific community, revolutionizing the way we separate and analyze complex mixtures. This versatile technique finds applications in a wide range of fields, from pharmaceuticals to environmental monitoring and food analysis.
Column Chromatography: The Foundation of HPLC
The heart of HPLC lies in column chromatography, a technique that separates components based on their differential interactions with a stationary phase packed within a column. As a sample flows through the column, components with varying affinities for the stationary phase are selectively retained or eluted, resulting in effective separation.
Mobile and Stationary Phases: Key Components of HPLC
The mobile phase, typically a liquid, carries the sample through the column. The selection of the mobile phase is crucial as it influences the solubility, partitioning, and elution of components. The stationary phase, often a solid or gel, provides the selective interaction with sample molecules, facilitating their separation.
Gradient Elution: Enhancing Separation Complexity
For complex mixtures containing substances with a broad range of affinities, gradient elution is employed. This technique gradually changes the composition or strength of the mobile phase over time, enabling the elution and separation of components that would otherwise co-elute under isocratic conditions.
Detection of Separated Components: The Role of Detectors
Various detectors are used in HPLC to monitor the presence of eluted components. These detectors, such as UV-Vis, fluorescence, and mass spectrometry, provide information on the concentration, identity, and properties of the separated compounds.
Peak Identification and Retention Time
The output of HPLC analysis is typically a chromatogram, which is a graphical representation of the retention time of individual components. Retention time, the time it takes for a component to elute from the column, is a key parameter in peak identification and quantification.
Applications of HPLC
HPLC has broad applications in various scientific fields, including:
- Pharmaceutical industry: Quality control, analysis of active ingredients, and drug development.
- Environmental monitoring: Detection of pollutants, water quality assessment, and soil analysis.
- Food analysis: Nutritional labeling, food safety, and authenticity testing.
HPLC is a versatile and powerful technique that has revolutionized the separation and analysis of chemical mixtures. Its principles of column chromatography, mobile and stationary phases, and detection techniques have made it an indispensable tool in various scientific disciplines, providing insights into the composition and properties of matter.
HPLC: A Journey into the World of Separation and Analysis
Imagine you’re a detective tasked with unraveling a complex puzzle—separating a mixture of different substances. Meet HPLC, your trusty tool, a high-performance liquid chromatography, that allows scientists to separate and identify even the most elusive components of a sample.
The Symphony of Column Chromatography and HPLC
HPLC is a dance between a column chromatography, where the mixture travels through a stationary phase, and a mobile phase, the solvent that carries the mixture. As the mixture moves through the column, different components interact with the stationary phase based on their unique properties. Lighter components dart through the column with ease, while heavier ones struggle to catch up. This interplay leads to the separation of components into distinct bands.
The Balancing Act of Mobile and Stationary Phases
The mobile phase and stationary phase are like partners in crime, each playing a vital role in the separation process. The mobile phase washes the mixture through the column at a controlled flow rate, while the stationary phase acts as a selective gatekeeper, allowing certain components to pass through while holding back others. By carefully choosing the composition and flow rate of the mobile phase, scientists can fine-tune the separation to achieve maximum efficiency.
Detecting the Hidden Treasures: The Magic of Detectors
Once separated, these distinct bands of components need to be detected. Enter the detectors, the sensory organs of HPLC. UV-Vis detectors detect components based on their ability to absorb light at specific wavelengths, while fluorescence detectors reveal components that emit light when exposed to certain frequencies. Each detector has its own unique superpowers, allowing scientists to identify and quantify even trace amounts of components.
Unveiling the Secrets: Chromatography’s Tale
The results of HPLC are displayed in a chromatogram, a graphical representation of the separation process. Each peak on the chromatogram represents a component in the mixture. The retention time of a peak—the time it takes for a component to travel through the column—is like its fingerprint, identifying it from its peers.
HPLC’s Limitless Potential: Unlocking Scientific Mysteries
HPLC is not just a technique; it’s a gateway to unlocking mysteries in various scientific fields. From unraveling the complexities of pharmaceuticals to identifying pollutants in the environment, and even ensuring the safety of our food, HPLC plays a pivotal role in scientific research and industry.
HPLC stands tall as an indispensable tool in the world of separation and analysis. Its ability to identify and quantify even the most elusive components has made it a workhorse in scientific laboratories across the globe. With its versatility and precision, HPLC empowers scientists to unravel the secrets of matter, paving the way for countless discoveries and advancements.
HPLC: A Powerful Tool for Separation and Purification in Science and Industry
In the realm of scientific research and industry, precision and accuracy are paramount. High-performance liquid chromatography (HPLC) stands as a cornerstone technique that empowers scientists and researchers to isolate, identify, and quantify compounds with unparalleled efficiency.
HPLC: The Foundation of Separation
Imagine a microscopic maze, where tiny particles embark on a journey through a labyrinth of obstacles. This is the essence of HPLC, a technique that separates compounds based on their unique properties. The maze, known as the column, is filled with a stationary phase, while the particles travel through a solvent called the mobile phase.
Mobile and Stationary Phases: The Symphony of Separation
The dance between the mobile and stationary phases is crucial in HPLC. As the mobile phase flows through the column, compounds interact with the stationary phase. Compounds that interact more strongly with the stationary phase are retained longer, while those that interact less move faster. This differential interaction is the key to separation.
Detecting the Separated Compounds: Unraveling the Chromatogram
Once separated, compounds must be detected and identified. HPLC employs a variety of detectors to accomplish this task. These detectors, like watchful sentinels, observe the separated compounds as they emerge from the column. Each detector is sensitive to specific characteristics, such as absorbance, fluorescence, or mass-to-charge ratio.
Peak Identification and Retention Time: The Fingerprint of Compounds
The chromatogram, a graphical representation of detector signals, unveils the fingerprints of separated compounds. Each peak on the chromatogram corresponds to a specific compound. The retention time, the time it takes for a compound to elute from the column, serves as a unique identifier.
Applications of HPLC: A Versatile Solution
HPLC’s versatility shines across a broad spectrum of applications. From pharmaceutical analysis to environmental monitoring, from food safety to biotechnology, HPLC provides invaluable insights into the composition and properties of complex mixtures.
HPLC is not merely a technique; it is an enabler of scientific discoveries and industrial advancements. Its precision and accuracy empower researchers to unravel the unknown, develop novel therapies, ensure food safety, and protect our environment. HPLC stands as a testimony to the transformative power of technology, driving innovation and shaping the future of science and industry.