The Interplay Of Matter And Energy: Exploring Fundamental Laws, Thermodynamics, And Relativity
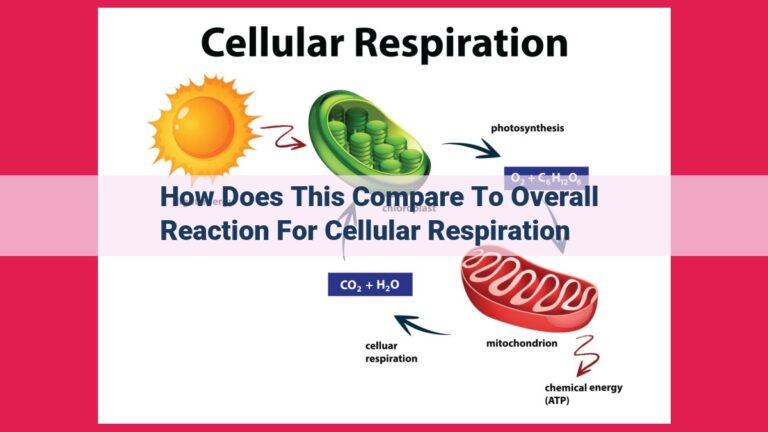
Matter and energy are interconnected through fundamental laws like the conservation of energy, which states that energy cannot be destroyed or created. The laws of thermodynamics describe energy transfer, transformation, and spontaneous processes. Properties like enthalpy and Gibbs free energy help quantify energy changes in various systems. Notably, relativity reveals the mass-energy equivalence, epitomized by Einstein’s equation E=mc², showcasing the deep connection between these two fundamental concepts.
Understanding the Conservation of Energy:
- Explain the Law of Conservation of Energy, emphasizing that energy cannot be created or destroyed, only transferred or transformed.
- Discuss energy transfer and transformation, providing examples of how energy flows within systems.
Understanding the Conservation of Energy: A Journey into the Energy Realm
In the vast expanse of the universe, amidst a symphony of cosmic processes, there exists a fundamental principle that governs the flow and transformation of energy: The Law of Conservation of Energy. This immutable law declares that energy cannot be created or destroyed, but it can be transferred or transformed from one form to another.
就像一位神奇的魔术师,能量在不同的形态之间穿梭, कभी गर्मी के रूप में प्रकट होती है, कभी प्रकाश के रूप में प्रकट होती है, और कभी गति के रूप में प्रकट होती है। इस निरंतर परिवर्तन के माध्यम से, ऊर्जा अपने अस्तित्व को बनाए रखती है, केवल अपने स्वरूप को बदलती है।
जैसे पानी एक नदी से दूसरी नदी में बहता है, ऊर्जा भी प्रणालियों के बीच यात्रा करती है। एक जलविद्युत बांध में, पानी की गतिज ऊर्जा को विद्युत ऊर्जा में बदल दिया जाता है, जिसे बाद में हमारे घरों को रोशन करने के लिए उपयोग किया जाता है। एक पेट्रोल इंजन में, रासायनिक ऊर्जा को यांत्रिक ऊर्जा में बदला जाता है, जो एक कार को गति में स्थापित करता है।
ये सिर्फ कुछ उदाहरण हैं कि कैसे ऊर्जा हमारे चारों ओर लगातार प्रवाहित होती है और बदलती है। इस रहस्यमय ऊर्जा के खेल को समझकर, हम अपने आसपास की दुनिया की सराहना कर सकते हैं और हमारे जीवन को बेहतर बनाने के तरीके खोज सकते हैं।
Exploring the Laws of Thermodynamics
In the realm of science, nothing is created or destroyed, and energy is no exception. This fundamental principle, known as the Law of Conservation of Energy, governs the flow and transformation of energy within all systems.
Within the framework of thermodynamics, two key laws provide a deeper understanding of energy’s behavior. Let’s delve into the First Law of Thermodynamics and its implications. This law states that within an isolated system, total energy remains constant. In other words, energy can neither be created nor destroyed, only transferred or transformed.
This concept has profound implications for energy transfers, heat flow, and work. Heat, which is the transfer of thermal energy, can either flow into or out of a system. Work, on the other hand, involves the transfer of energy through non-thermal means, such as mechanical or electrical processes. The First Law dictates that any changes in internal energy within a system must be accounted for by either heat transfer or work.
Now, let’s turn our attention to the Second Law of Thermodynamics, which introduces the intriguing concept of entropy. Entropy, simply put, is a measure of disorder or randomness within a system. This law states that the entropy of an isolated system always increases over time, a phenomenon known as the increase of entropy.
This fundamental principle explains the irreversibility of many natural processes. For instance, heat naturally flows from hotter to colder regions, never the other way around. This _unidirectional** flow of energy is driven by the relentless increase in entropy.
By understanding these fundamental laws of thermodynamics, we gain invaluable insights into the behavior of energy in our universe. These laws govern everything from the operation of heat engines to the workings of biological systems. Embracing the principles of thermodynamics empowers us to harness energy efficiently, respect its transformative power, and appreciate the subtle dance of order and disorder that shapes our world.
Delving into the Enigmatic Properties of Matter and Energy
In the realm of physics, energy and matter intertwine in a fascinating dance, governed by intricate principles that shape our world. Among these principles lie enthalpy and Gibbs Free Energy, concepts that unlock the secrets of heat capacity and thermodynamic equilibrium.
Enthalpy: A Measure of Heat Exchange
Enthalpy, symbolized by H, represents the total thermal energy of a system, capturing both its internal energy and the work it can do on its surroundings. It measures the heat absorbed or released as a system undergoes changes in temperature, pressure, or volume. Enthalpy plays a crucial role in understanding heat capacity, which quantifies a substance’s ability to absorb heat without undergoing significant temperature changes.
Gibbs Free Energy: Equilibrium and Maximum Work
Gibbs Free Energy, denoted by G, is a thermodynamic potential that combines enthalpy and entropy, providing insights into the maximum work that a system can perform at constant temperature and pressure. It serves as a criterion for spontaneity, indicating whether a reaction or process will proceed spontaneously or require external input. A negative change in Gibbs Free Energy signifies a spontaneous process.
By understanding these properties, scientists can delve into the intricacies of chemical reactions, phase transitions, and energy transfer. Enthalpy and Gibbs Free Energy illuminate the behavior of matter and energy, guiding researchers toward advancements in fields such as materials science and energy conversion.
The Mass-Energy Equivalence: A Tale of Energy and Matter
In the realm of physics, an extraordinary discovery emerged, forever altering our perception of mass and energy. The concept of relativity, proposed by Albert Einstein, shattered the conventional understanding of separate entities and introduced the profound connection between these fundamental properties.
Einstein’s groundbreaking equation, E=mc², unveiled the mass-energy equivalence. It revealed that mass possesses an extraordinary property: the capacity to be converted into energy and vice versa. This equation has profound implications for our comprehension of the universe and the very essence of existence.
The implications of the mass-energy equivalence are far-reaching, extending from the subatomic realms of nuclear reactions to the colossal scale of stellar processes. Nuclear energy, harnessed through nuclear power plants, provides a testament to the power of converting mass into usable energy. The process of nuclear fission, the splitting of atomic nuclei, exemplifies this conversion, releasing immense amounts of energy in the form of heat and radiation.
Einstein’s equation not only revolutionized our understanding of mass and energy but also opened doors to remarkable technological advancements. It laid the groundwork for the development of nuclear weapons, a sobering reminder of the destructive potential of unchecked scientific progress. However, it also paved the way for peaceful applications, such as the generation of nuclear power, which provides a significant proportion of the world’s electricity.