Mastering Solubility Rules: An Effective Guide For Memorization
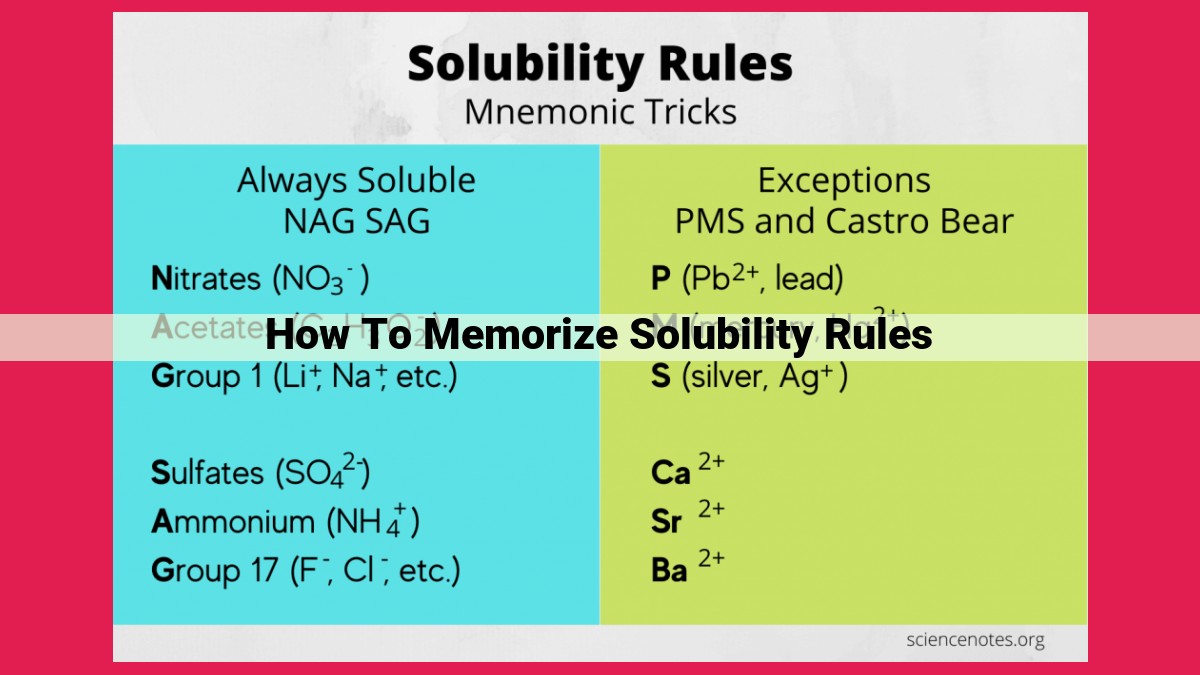
To memorize solubility rules effectively, break them down into categories. Start with the basic concepts of anions and cations. Remember that Group 1 and Group 2 cations are generally soluble. Ammonium (NH4+) and nitrate (NO3-) ions are also soluble. For anions, focus on the common ones like chloride (Cl-), bromide (Br-), and iodide (I-). They form soluble salts with most cations. Polyatomic anions like sulfate (SO42-), carbonate (CO32-), and phosphate (PO43-) require more attention. Use mnemonic devices like rhymes or stories to make memorizing these rules more enjoyable and effective.
Memorizing Solubility Rules: A Comprehensive Guide
Understanding solubility rules is crucial for comprehending chemical reactions and ion behavior. These rules predict whether ionic compounds dissolve in water, forming ions or remaining intact as insoluble solids. This blog post will guide you through a structured approach to memorizing solubility rules, making your journey into the world of chemistry more manageable.
Basic Concepts: Ions and Molecules
Before delving into solubility rules, let’s revisit the fundamental concepts of ions and molecules. Ions are atoms or groups of atoms that have gained or lost electrons, resulting in a net electrical charge. Cations are positively charged ions, while anions are negatively charged ions.
Solubility Rules
Solubility rules are generalizations that predict the solubility of ionic compounds in water. The most common rules are:
- Group 1 cations (Li+, Na+, K+, Rb+, Cs+) and ammonium ion (NH4+) are soluble.
- Group 2 cations (Ca2+, Sr2+, Ba2+) are soluble except for sulfate and carbonate salts.
- Nitrate (NO3-), chloride (Cl-), bromide (Br-), iodide (I-), and hydroxide (OH-) ions form soluble salts with all cations.
- Sulfate (SO42-) and phosphate (PO43-) ions form insoluble salts with calcium, strontium, and barium cations.
- Carbonate (CO32-) ions form insoluble salts with all cations except for sodium, potassium, and ammonium.
Exceptions and Factors Affecting Solubility
While these rules provide a general framework, certain exceptions and factors can affect solubility:
- Temperature and pressure can influence solubility.
- pH can affect the solubility of certain compounds, such as carbonates and hydroxides.
- Common ion effect occurs when adding a common ion to a solution, which decreases the solubility of a sparingly soluble salt.
Mnemonic Devices
Mnemonic devices are memory aids that can help you recall solubility rules more easily. One popular mnemonic for solubility rules is:
“Sally Can Bring Ice Cold Beer On Friday Night And Hand It Over.”
- Sally (soluble)
- Can (cations)
- Bring (bromide, iodide)
- Ice (insoluble)
- Cold (carbonate)
- Beer (barium, strontium)
- On (nitrate)
- Friday (fluoride)
- Night (nitrite)
- And (ammonium)
- Hand (hydroxide)
- It (iodate)
- Over (oxalate)
By understanding these solubility rules and employing mnemonic devices, you can develop a solid foundation in predicting the behavior of ions in aqueous solutions. This knowledge is essential for understanding various chemical reactions and processes. Remember, the key to memorizing solubility rules lies in consistent practice and using techniques that enhance your memory.
Anions and Cations: The Basic Building Blocks of Chemistry
Anions and cations are the two types of ions, electrically charged atoms or molecules. They are the fundamental building blocks of ionic compounds, which are formed when positively charged cations combine with negatively charged anions.
Anions are negatively charged ions because they have gained one or more electrons. They typically end in “-ide” or “-ate,” such as chloride (Cl-) or sulfate (SO42-). Anions often form from nonmetallic elements.
Cations, on the other hand, are positively charged ions because they have lost one or more electrons. They commonly end with “-ium” or “+,” such as sodium (Na+) or calcium (Ca2+). Cations are usually formed from metallic elements.
The charge of an ion is determined by the number of electrons it has gained or lost. For example, chloride has gained one electron, so it has a charge of -1. Calcium has lost two electrons, so it has a charge of +2.
Ionic compounds are formed when cations and anions combine in such a way that their charges balance each other out. For example, sodium chloride (NaCl) is formed when sodium ions (Na+) combine with chloride ions (Cl-). The sodium ion has a +1 charge, and the chloride ion has a -1 charge, so the overall charge of the compound is zero.
Solubility Rules for Specific Ions
Understanding the solubility rules for ionic compounds is crucial in chemistry, allowing us to predict the behavior of ions in solution. Let’s dive into the fundamental rules and explore the exceptions that can affect solubility.
General Solubility Rules
In water, most ionic compounds dissolve to form ions. The general solubility rules help us predict which compounds will dissolve and which will form a precipitate (a solid that forms when two solutions react). These rules include:
- All Group 1 cations (Li+, Na+, K+, Rb+, Cs+) and the ammonium ion (NH4+) are soluble.
- All Group 2 cations (Ca2+, Sr2+, Ba2+) are soluble, except for BaSO4.
- All nitrate (NO3-) and chloride (Cl-) ions are soluble.
- All bromide (Br-), iodide (I-), sulfate (SO42-), carbonate (CO32-), and phosphate (PO43-) ions are soluble, except when combined with specific cations like Ba2+ or Ca2+.
Exceptions to the Rules
Certain factors can influence the solubility of ionic compounds, including:
- Temperature: Increased temperature generally increases solubility.
- Pressure: Increased pressure may decrease solubility for gases.
- pH: Changes in pH can affect the solubility of certain compounds, especially those involving weak acids or bases.
Complexation
In some cases, ions can form complexes with other molecules or ions, which can alter their solubility. For example, the addition of ammonia (NH3) to a solution containing copper(II) ions (Cu2+) forms a complex ion [Cu(NH3)4]2+, which is more soluble than Cu2+.
Applications
Solubility rules have practical applications in many areas, including:
- Chemistry: Predicting reaction products and solubility of substances.
- Environmental science: Understanding water quality and pollution.
- Pharmacology: Designing drugs that can dissolve and be absorbed by the body.
Exceptions to the Solubility Rules: Unraveling the Factors that Alter Solubility
While solubility rules provide a general framework for predicting the solubility of ionic compounds in water, exceptions to these rules exist, complicating the understanding of chemical reactions and ion behavior. Temperature, pressure, and pH play significant roles in altering the solubility of ionic compounds, making it crucial to consider these factors in chemical processes.
Temperature: As temperature increases, the solubility of most ionic compounds increases. This is because the higher kinetic energy of the solvent molecules at higher temperatures allows them to overcome the solute-solute interactions that hold the solid compound together. However, for some ionic compounds, solubility decreases with increasing temperature. This occurs when the hydration of ions (the attraction of water molecules to the ions) becomes less favorable at higher temperatures, leading to a decrease in solubility.
Pressure: Unlike temperature, pressure has a minimal effect on the solubility of most ionic compounds in water. However, for gases that can dissolve in water, such as carbon dioxide, increased pressure increases solubility. This is because the partial pressure of the gas above the solution increases, forcing more gas molecules into the solution.
pH: The pH of a solution can significantly alter the solubility of ionic compounds. For example, the solubility of metal hydroxides (compounds containing the OH- ion) decreases with increasing pH. This occurs because the OH- ions in the solution compete with the metal ions for water molecules, reducing the hydration of the metal ions and promoting precipitation.
The common ion effect is another important exception to the solubility rules. When a common ion, an ion that is present in both the растворимое соединение and the added electrolyte, is added to a solution, it decreases the solubility of the растворимое соединение. This is because the common ion competes with the ions of the растворимое соединение for water molecules, reducing their hydration and promoting precipitation.
Group 1 Cations: The Alkali Metals
In the realm of chemistry, ions—charged particles—play a crucial role in understanding the behavior of various substances. Cations specifically, are positively charged ions, and among them, the Group 1 cations stand out as a fascinating group.
Group 1 cations, fondly known by the symbols Li+, Na+, K+, Rb+, and Cs+, belong to a family of elements known as alkali metals. These elements share a common characteristic: they readily form compounds by losing one electron to achieve a stable octet configuration.
The alkali metals are highly reactive, eager to give up their lone electron to form ionic bonds with other elements. This reactivity increases as we move down the group, making cesium (Cs) the most reactive of all.
Alkali metal ions are all 1+ ions with identical charges. This uniform charge reflects their single valence electron. Their positive charge attracts negatively charged ions, forming ionic compounds. For instance, sodium reacts with chlorine to form sodium chloride (NaCl), a common salt that enhances the flavor of our meals.
To summarize, Group 1 cations are the positively charged ions of alkali metals. These elements have a single valence electron that they readily lose, giving them a +1 charge and making them highly reactive. They form ionic bonds with other elements, creating compounds like sodium chloride, which play a significant role in various chemical reactions and everyday life.
Group 2 Cations (Ca2+, Sr2+, Ba2+)
- Explain the alkaline earth metals, their reactivity, and their properties as 2+ ions.
- Relate to the concept of alkaline earth metals and their chemical properties.
Group 2 Cations: Unveiling the Alkaline Earth Metals
Embark on an adventure into the realm of chemistry as we unravel the mysteries of Group 2 cations: _Ca2+, Sr2+_, and _Ba2+_. These remarkable ions belong to a group of elements known as the alkaline earth metals, renowned for their exceptional reactivity.
Imagine these metals as eager dancers, swiftly shedding electrons to form stable 2+ ions. They dance with a fiery intensity, reacting vigorously with water to produce hydrogen gas and hydroxide ions. This passionate reaction underscores their basic nature and their pivotal role in countless chemical processes.
But these ions hold more surprises. They possess a unique affinity for oxygen, forming stable oxides that find widespread use in construction and metallurgy. Calcium oxide, for instance, plays a crucial role in the production of cement, while strontium oxide lends its luminescent properties to fireworks.
Furthermore, Group 2 cations exhibit a strong attraction to sulfate ions, leading to the formation of sparingly soluble sulfates. These compounds find applications in the production of paper, paint, and plaster.
So, let’s raise a glass to the alkaline earth metals, the behind-the-scenes heroes of countless chemical reactions. Their unique properties make them indispensable in a wide range of industries, from construction to medicine. Remember, these elements are the keystone that unlocks the secrets of ion behavior and chemical reactivity.
Ammonium Ion (NH₄⁺): Understanding Its Unique Properties
Welcome to the fascinating world of polyatomic ions, where we’ll explore the intriguing ammonium ion (NH₄⁺). This ion is a ubiquitous presence in many chemical reactions, and understanding its properties is essential for navigating the complexities of chemistry.
The **polyatomic nature of ammonium makes it distinct from its monatomic counterparts. It consists of a combination of nitrogen and hydrogen atoms, forming a charged molecule. Its unique structure and properties make it behave differently from other ions.
Ammonium, like other polyatomic ions, is composed of multiple atoms covalently bonded together. These covalent bonds distribute the charge across the molecule, resulting in a more stable and less reactive ion. The positive charge of the ammonium ion is evenly distributed over the four hydrogen atoms, giving it a symmetrical and stable structure.
Equilibrium reactions play a significant role in understanding the behavior of ammonium ions. These reactions involve the reversible exchange of protons between ammonium ions and water molecules, establishing an equilibrium between the two forms. The extent to which this equilibrium shifts can affect the pH of solutions and influence other chemical reactions.
Acids and bases are closely intertwined with ammonium ions. Ammonium ions are conjugate acids of the weak base ammonia (NH₃). Understanding the equilibrium between ammonium ions and ammonia is crucial for comprehending acid-base reactions and their impact on pH.
In summary, the ammonium ion (NH₄⁺) is a polyatomic ion with a unique structure and properties. Its polyatomic nature, covalent bonding, and involvement in equilibrium reactions make it an essential player in various chemical processes. By understanding the properties of ammonium ions, we can better appreciate the intricate dance of ions and molecules in the fascinating world of chemistry.
Nitrate Ion: The Versatile Nitrogen Compound
The nitrate ion (NO3-) is a fascinating polyatomic ion that plays a crucial role in various chemical processes. Its unique properties and wide applications make it an essential topic in chemistry.
Properties of Nitrate Ion:
The nitrate ion is composed of one nitrogen atom and three oxygen atoms. It has a -1 charge, making it a highly reactive species. Nitrate ions are colorless, odorless, and form stable salts with many metals. Their high solubility in water contributes to their widespread presence in both natural and man-made environments.
Related Concepts:
-
Nitrogen Compounds: Nitrate ion is a member of the nitrogen compound family, which also includes ammonia, nitric acid, and nitrous oxide. These compounds exhibit a diverse range of properties and are essential for life processes.
-
Oxidizing Agents: Nitrate ions have mild oxidizing properties. This means they can accept electrons from other species, causing their reduction. This property finds applications in analytical chemistry and industrial processes.
-
Fertilizers: Nitrates are indispensable in agriculture as they provide nitrogen, a vital nutrient for plant growth. Commercial fertilizers often contain nitrate salts, such as ammonium nitrate or potassium nitrate, to supplement soil nitrogen levels.
Significance in Various Fields:
Nitrate ion has far-reaching implications in different fields:
-
Water Quality: Nitrate contamination in water sources can pose health risks, as high nitrate levels can lead to methemoglobinemia, a condition in which the oxygen-carrying capacity of blood is reduced.
-
Environmental Science: Nitrate pollution from agricultural runoff and industrial effluents can disrupt aquatic ecosystems, promote algal blooms, and contribute to eutrophication.
-
Food Preservation: Nitrates are used as preservatives in cured meats to prevent the growth of harmful bacteria.
The nitrate ion is a versatile and significant polyatomic ion with diverse applications. Its unique properties, related concepts, and far-reaching implications highlight its importance in various scientific disciplines. Understanding the nitrate ion’s behavior and reactivity is essential for chemists, environmentalists, and individuals concerned with health and food safety.
Unlocking the Secrets of Chloride Ions: A Comprehensive Guide
In the realm of chemistry, understanding the solubility rules of ions is crucial for unraveling the mysteries of chemical reactions and unraveling the enigmatic behavior of ions in solution. Among these ions, the chloride ion, denoted as Cl-*, stands out as a captivating subject that deserves our undivided attention.
Defining the Chloride Ion: A Tiny Player with a Mighty Presence
The chloride ion is a monatomic ion, a single negatively charged atom, belonging to the halogen family. This family of elements shares a common trait of high reactivity and a voracious appetite for electrons. Chlorine, in its elemental form, is a pale green gas that plays a vital role in various industrial processes and everyday life.
Exploring the Properties of Chloride Ions: Salt, the Spice of Life
Chloride ions are ubiquitous in nature, often forming ionic compounds with various metals. The most well-known of these compounds is sodium chloride, commonly known as table salt, an essential ingredient that adds flavor to our meals and preserves food. Chloride ions also participate in physiological processes, contributing to the proper functioning of our bodies.
Chloride Ions in the Dance of Chemistry: Reactivity and Ionic Bonding
The reactivity of chloride ions stems from their tendency to form ionic bonds with positively charged ions. This ability to form strong bonds makes chloride ions essential players in a multitude of chemical reactions. In aqueous solutions, chloride ions readily dissolve, forming hydrated ions surrounded by water molecules.
Delving into the World of Halogens: A Family of Reactive Elements
The chloride ion is an integral member of the halogen family, a group of elements that includes fluorine, bromine, and iodine. These elements share a common property of being highly electronegative, meaning they have a strong affinity for electrons. This electronegativity drives their reactivity and makes them essential components in various chemical processes.
Understanding the properties and behavior of chloride ions unlocks a deeper comprehension of chemical reactions and ionic interactions. From their role in forming ionic compounds like table salt to their participation in physiological processes, chloride ions play a multifaceted role in the world around us. By mastering the solubility rules, we gain a valuable tool for predicting the behavior of these enigmatic particles and unraveling the secrets of chemical systems.
Bromide Ion (Br)
In the realm of chemistry, understanding the behavior of ions is crucial for predicting the solubility of ionic compounds. Among these ions, the bromide ion (Br) stands out as an intriguing player.
Bromide is a monatomic ion derived from the halogen element bromine, which belongs to Group 17 of the periodic table. It carries a negative charge of one and forms ionic bonds with positively charged ions, resulting in the formation of ionic compounds.
Bromine itself is a volatile liquid with a pungent odor. In its elemental form, it is highly reactive and combines readily with other elements. Upon reacting with metals, it forms ionic compounds known as bromides.
In water, bromide ions dissolve to form a transparent solution that is colorless and odorless. Bromide is soluble in water because it forms hydrated ions, which are surrounded by water molecules. This interaction between bromide ions and water molecules makes it difficult for the ions to come together and form an insoluble solid.
Bromine is a relatively unreactive element compared to its halogen counterparts, chlorine and iodine. This is because the bromide ion is less reactive than the chloride and iodide ions. As a result, bromide compounds are generally more stable and less likely to undergo chemical reactions.
Despite its lower reactivity, bromine does exhibit some unique properties. For instance, it displaces chloride ions in aqueous solutions, forming bromide ions and chloride gas. This reaction is commonly used to test for the presence of bromide ions in a solution.
Iodide Ion (I-)
Prepare to delve into the realm of chemistry as we explore the captivating world of the iodide ion, a fascinating and intriguing component of the chemical landscape. Our journey begins with a fundamental understanding of its atomic structure and essential properties, laying the groundwork for unraveling its role in shaping chemical reactions and biological processes.
Properties of the Iodide Ion:
The iodide ion, denoted by the chemical symbol I-, is a monatomic ion, meaning it consists of a single iodine atom. Its negative charge arises from the presence of an extra electron, bestowing upon it unique chemical characteristics. Iodine, a non-metallic element, belongs to the halogen group, a family of highly reactive elements that includes chlorine, bromine, and fluorine.
Chemical Interactions:
Iodide ions readily participate in various chemical reactions, exhibiting a strong affinity for forming ionic compounds with positively charged ions, known as cations. These ionic compounds, such as potassium iodide (KI) or sodium iodide (NaI), are highly soluble in water, contributing to their widespread presence in aqueous solutions.
Biological Significance:
Beyond the realm of chemistry, iodide ions play a crucial role in biological systems. They are essential for the proper functioning of the thyroid gland, a small organ responsible for regulating metabolism and growth. Iodine deficiency can lead to various health conditions, including hypothyroidism and goiter.
Applications in Various Fields:
The iodide ion finds practical applications across diverse fields. In photography, silver iodide serves as a light-sensitive material in photographic film, capturing images through a process known as photolysis. In medicine, radioactive iodine isotopes, such as iodine-131, are utilized in medical imaging and radiation therapy for treating thyroid disorders and certain types of cancer.
Through this exploration of the iodide ion, we have gained invaluable insights into its chemical properties, biological significance, and practical applications. Its unique characteristics have made it an indispensable component in various scientific and industrial endeavors, highlighting the multifaceted nature of chemistry and its profound impact on our daily lives.
Sulfate Ion (SO₄²⁻) – The Versatile Building Block of Life
In the realm of chemistry, the sulfate ion plays a crucial role as a polyatomic building block, forming the foundation of numerous compounds that are essential to life. This extraordinary ion consists of one sulfur atom covalently bonded to four oxygen atoms, creating a stable tetrahedral structure.
The sulfate ion’s properties extend beyond its basic structure. It acts as a non-toxic and odorless substance, making it safe to handle and use in various applications. Additionally, it is a strong oxidizing agent, capable of oxidizing other substances and facilitating a wide range of chemical reactions.
In nature, the sulfate ion is abundant, occurring naturally in minerals such as gypsum (CaSO₄·2H₂O) and Epsom salt (MgSO₄·7H₂O). It is also present in seawater and plays a vital role in the biological processes of plants and animals. For instance, it serves as a source of sulfur for the synthesis of proteins and is involved in cellular respiration.
Moreover, the sulfate ion finds extensive use in various human activities. It is employed in the manufacturing of detergents, paper, and glass, among other products. In the agricultural industry, it is used as a fertilizer, providing essential sulfur for optimal plant growth.
In summary, the sulfate ion is a versatile and ubiquitous substance with a wide range of properties and applications. Its stability, oxidizing nature, and abundance make it an invaluable component in both the natural and industrial worlds. Whether it’s supporting the growth of plants or facilitating industrial processes, the sulfate ion continues to play a critical role in our daily lives.
Carbonate Ion (CO32-): A Vital Polyatomic Ion in Chemistry
In the realm of chemistry, solubility rules play a crucial role in predicting the behavior of ionic compounds in water. Among these ions, the carbonate ion (CO32-) stands out as a polyatomic ion with unique properties and a wide range of applications.
Understanding Polyatomic Ions
Polyatomic ions are groups of atoms that carry an overall charge, making them act as a single unit. The carbonate ion is composed of one carbon atom and three oxygen atoms, bonded together with a negative charge of 2.
Properties of Carbonate Ion
Insoluble in Water: Carbonates are generally insoluble in water, forming precipitates when combined with certain cations. This phenomenon is essential in various chemical processes, including precipitation reactions and the formation of carbonate minerals.
Weak Base: Carbonate ion also acts as a weak base, reacting with water to form carbonic acid (H2CO3). This reaction plays a crucial role in maintaining the pH balance in aquatic environments.
Related Concepts
- Carbon Compounds: Carbonates are closely related to other carbon compounds, such as carbon dioxide (CO2) and organic molecules containing carbonate groups.
- Carbonic Acid: The reaction of carbonate ion with water produces carbonic acid, a weak acid that readily dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-).
- Properties of Carbonates: Carbonates, as a class of compounds, exhibit a range of properties, including low solubility, high thermal stability, and the ability to form insoluble salts with certain cations.
Phosphate Ion (PO₄³⁻): Understanding Its Properties and Behavior
Embarking on a journey to comprehend the intricate world of chemistry, we encounter the fascinating realm of solubility rules. These rules govern the ability of ionic compounds to dissolve in water, shaping chemical reactions and the behavior of ions. Among these essential rules lies the solubility of the phosphate ion (PO₄³⁻), an intriguing polyatomic ion with unique properties and fascinating connections to everyday life.
Unveiling the Phosphate Ion
At the heart of the phosphate ion resides a central phosphorus atom, surrounded by four oxygen atoms arranged in a tetrahedral configuration. This polyatomic ion carries a negative charge of -3, owing to the three extra electrons present in its structure. Its stability and prevalence in nature make it a cornerstone of countless chemical and biological processes.
Phosphorus Compounds and Their Significance
Phosphorus, the element at the heart of the phosphate ion, plays a pivotal role in the realm of biochemistry. It serves as a key component in phosphorus compounds, such as DNA and RNA, the blueprints of life that carry genetic information. Additionally, phosphates are essential for energy transfer within cells, fueling biological processes through molecules like ATP.
Phosphoric Acid and Its Properties
The phosphate ion’s connection to phosphorus compounds extends to phosphoric acid (H₃PO₄), a colorless, odorless acid commonly used in industrial settings. Phosphoric acid is a versatile substance, employed in the production of fertilizers, detergents, and food additives. Its acidity and the presence of multiple hydrogen ions (H+) make it an excellent chelating agent, capable of binding to metal ions and forming stable complexes.
Solubility and Precipitation
The solubility of the phosphate ion in water depends on the nature of the cation it is paired with. In general, phosphates of alkali metals (Group 1) and ammonium (NH₄⁺) are soluble, meaning they readily dissolve in water and form clear solutions. However, phosphates of alkaline earth metals (Group 2), such as calcium (Ca²⁺) and barium (Ba²⁺), are insoluble in water, forming solid precipitates. This precipitation behavior is a crucial concept in analytical chemistry, enabling the separation and identification of ions in solution.
Unraveling the Hydroxide Ion: The Key to Understanding Bases
In the realm of chemistry, understanding the solubility of ionic compounds is crucial for predicting chemical reactions and ion behavior. Among these ions lies the hydroxide ion (OH-), a monatomic ion that plays a pivotal role in shaping the properties of bases.
Meet the Hydroxide Ion: A Monatomic Force
The hydroxide ion is a single negatively charged ion with a chemical formula of OH-. It forms when water molecules (H2O) undergo ionization, resulting in the release of a hydrogen ion (H+) and a hydroxide ion. This ionization process is at the heart of many chemical reactions that involve bases.
Bases and the Hydroxide Ion: A Symbiotic Relationship
Hydroxide ions are synonymous with bases. A base is a substance that can donate hydroxide ions to a solution. When a base is dissolved in water, it dissociates, releasing hydroxide ions into the solution. This increase in hydroxide ion concentration leads to a higher pH, indicating a more basic solution.
Neutralization Reactions: A Dance of Ions
Hydroxide ions are also central to neutralization reactions, where an acid and a base react to form a salt and water. In these reactions, the hydrogen ions from the acid combine with the hydroxide ions from the base to form water molecules. This process neutralizes the solution, resulting in a pH closer to neutral.
pH and the Hydroxide Ion: A Balancing Act
The concentration of hydroxide ions in a solution directly affects its pH. The pH scale measures the acidity or basicity of a solution, with a pH of 7 being neutral. Solutions with a pH below 7 are acidic, while solutions with a pH above 7 are basic. Hydroxide ions play a crucial role in maintaining a solution’s pH by counteracting the effects of hydrogen ions.
Mastering Solubility Rules with Fun and Effective Mnemonics
In the realm of chemistry, understanding solubility rules is paramount for predicting the behavior of ions in various solutions. Memorizing these rules can be daunting, but fear not, for we have a secret weapon – mnemonic devices. These ingenious tools employ creativity and imagination to transform complex information into unforgettable nuggets of knowledge.
What are Mnemonic Devices?
Mnemonics are memory aids that leverage various techniques to enhance recall. They work by associating new information with something already familiar, creating mental shortcuts that make memorization effortless.
Unleashing the Power of Mnemonics for Solubility Rules
Let’s explore some effective mnemonics specifically designed to help you conquer the solubility rules:
-
Acronyms and Acrostics: Create catchy acronyms or acrostics that represent the ions and their solubility properties. For instance, “CAST NaP” (Carbonate, Ammonium, Sodium, Potassium) for compounds that are soluble in water.
-
Songs and Rhymes: Compose memorable tunes or rhymes that incorporate the solubility rules. One example is the “Solubility Rap”:
“Alkali metals, cations are one,
Met with anions, they’re soluble, son.
Halides, nitrates, all dissolve like glue,
But carbonates and phosphates, they won’t do.”
-
Visual Aids: Create mind maps or colorful diagrams that visually depict the solubility rules. Use different colors or symbols to represent different ions and their behavior.
-
Storytelling and Analogies: Craft captivating stories or draw analogies that connect the solubility rules to familiar concepts. For instance, compare the “insoluble” sulfates to stubborn teenagers who refuse to mix with water.
-
Chunking and Spaced Repetition: Break down the solubility rules into smaller chunks and review them at spaced intervals. This repetition reinforces the information in your memory.
Embrace the Fun and Reaping the Rewards
By incorporating these mnemonic devices into your study routine, you can transform the task of memorizing solubility rules into an enjoyable and effective experience. Embrace the fun and reap the rewards of enhanced memory and a deeper understanding of chemical reactions. Remember, chemistry doesn’t have to be a daunting subject – with a little creativity and the power of mnemonics, you can conquer the world of solubility rules!