Mastering Rate Constant Calculations: Integrated Rate Laws And Half-Life Analysis
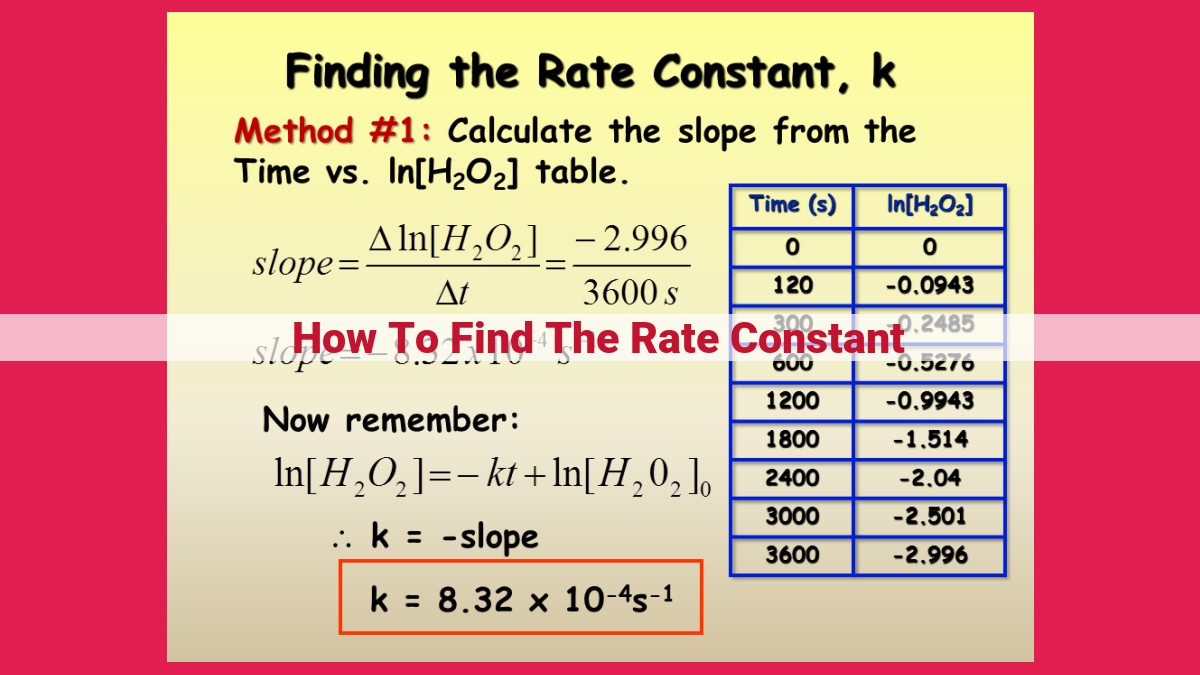
Using integrated rate laws or half-life measurements, the rate constant (k) can be calculated. Integrated rate laws provide an equation that relates reactant concentrations to time, with k being the slope or exponent. Half-life (t1/2), defined as the time for half of the reactants to react, also relates to k and reaction order. Arrhenius equation, k = Ae^(−Ea/RT), links k with activation energy (Ea), temperature (T), and a pre-exponential factor (A).
Understanding Reaction Orders:
- Define zero-order, first-order, and second-order reactions.
- Explain how reaction order affects the rate of the reaction.
Understanding Reaction Orders: The Key to Unlocking Reaction Rates
In the realm of chemistry, reaction orders play a crucial role in determining the rate at which reactions proceed. Understanding reaction orders is like having a compass in the world of chemical reactions, guiding us toward predicting and controlling their speed.
Defining Reaction Orders
A reaction order represents the power to which the concentration of a reactant is raised in the rate law equation. We encounter three common reaction orders:
- Zero-order reaction: The rate of the reaction is independent of the concentration of any reactants.
- First-order reaction: The rate of the reaction is directly proportional to the concentration of one reactant.
- Second-order reaction: The rate of the reaction is proportional to the square of the concentration of one reactant or the product of the concentrations of two reactants.
Influence on Reaction Rate
The reaction order has a profound impact on the reaction rate. For example, in a first-order reaction, doubling the concentration of the reactant will double the reaction rate. In contrast, in a second-order reaction, doubling the concentration will quadruple the rate.
By understanding reaction orders, chemists can make informed predictions about how altering reactant concentrations will affect reaction rates. This knowledge is essential for optimizing chemical processes and designing reactions that meet specific requirements.
Integrated Rate Laws:
- Define integrated rate laws.
- Discuss how they are used to determine reaction order and calculate the rate constant.
Understanding Integrated Rate Laws: A Guide for Chemical Explorers
Integrated rate laws are essential tools in the realm of chemical kinetics, allowing us to unravel the mysteries of reaction rates. These mathematical equations provide a comprehensive description of how a reaction’s concentration changes over time, helping us understand the reaction’s dependencies on various factors like temperature and concentration.
Journey into the World of Rate Orders
An integrated rate law’s most fundamental role lies in determining the reaction order. This crucial parameter reveals how the reaction rate varies with respect to the concentration of the reactants. By studying the integrated rate law, we can classify reactions as zero-order, first-order, or second-order.
Unveiling the Rate Constant: The Key to Reaction Speed
The integrated rate law doesn’t only unveil the reaction order; it also grants us access to a precious piece of information: the rate constant (k). This constant serves as a measure of the reaction’s speed and gives us insights into the inherent properties of the reaction itself.
Connecting Concepts: Units and Dimensions
Depending on the reaction order, the rate constant assumes different units. For zero-order reactions, it’s simply a constant, while for first-order reactions, it carries units of inverse time (e.g., s^-1). Understanding these units is crucial for interpreting the rate constant’s magnitude and comparing it across different reactions.
Embark on the Arrhenius Adventure
The Arrhenius equation, a vital component in chemical kinetics, establishes a profound link between the rate constant (k) and two key factors: activation energy (Ea) and temperature (T). Ea represents the minimum energy molecules must overcome to react, while temperature governs the average molecular energy.
Activation Energy: The Gateway to Chemical Reactions
Activation energy plays a pivotal role in determining the reaction rate. Higher activation energies necessitate more energetic collisions, resulting in slower reactions. In contrast, lower activation energies allow for more frequent successful collisions, leading to faster reactions.
The Temperature-Rate Constant Dance
Temperature exerts a profound influence on reaction rates. According to the Arrhenius equation, as temperature increases, the rate constant exponentially increases. This relationship signifies that reactions proceed faster at higher temperatures.
The Takeaway: A Deeper Understanding of Reaction Dynamics
Integrated rate laws, coupled with the Arrhenius equation, provide a comprehensive framework for understanding reaction dynamics. From determining reaction order and calculating rate constants to uncovering the influence of activation energy and temperature, these concepts empower us to unravel the intricate dance of chemical reactions and predict their behavior under different conditions.
Understanding Reaction Kinetics: The Significance of Half-Life and Rate Constants
In the realm of chemistry, understanding reaction kinetics is crucial to comprehending how chemical reactions occur and the factors that influence their speed. Among these factors, half-life and rate constant play significant roles.
Half-Life: A Measure of Reaction Progress
Imagine you have a bowl of apples that spoil over time. The half-life of the apples represents the time it takes for half of them to spoil. Similarly, in chemical reactions, half-life is the time it takes for half of the reactants (the initial substances) to be consumed or converted into products (the final substances).
Half-life serves as a diagnostic tool to determine the order of a reaction. Zero-order reactions have a constant half-life, first-order reactions have a half-life that is independent of [reactant] concentration, and second-order reactions have a half-life that is inversely proportional to [reactant] concentration.
Rate Constant: A Measure of Reaction Speed
The rate constant is a numerical value that quantifies how quickly a reaction occurs. It represents the proportionality between the rate of the reaction and the concentration of the reactants. A higher rate constant indicates a faster reaction.
The units of the rate constant depend on the order of the reaction. For zero-order reactions, the rate constant has units of [concentration]/[time], for first-order reactions, it is simply [1/time], and for second-order reactions, it is [1/([concentration][time])].
By combining half-life and rate constant, we can develop a deeper understanding of reaction kinetics. The half-life provides insight into the temporal aspect of a reaction, while the rate constant quantifies the speed of the reaction. Together, they help us unravel the dynamics of chemical reactions.
Rate Constant: A Measure of Reaction Speed
In chemical reactions, the rate constant is a critical parameter that quantifies the speed of the reaction. Imagine a race, where the rate constant is the velocity of a car representing the reaction’s progress. A higher rate constant means the car (reaction) moves faster, leading to a quicker reaction.
The significance of the rate constant lies in its ability to predict the rate of a reaction under specific conditions. It’s like knowing the car’s speed to estimate how quickly it will reach the finish line. By understanding the rate constant, scientists can design experiments and optimize reactions for desired outcomes.
Units of Rate Constant:
The units of rate constants vary depending on the order of the reaction. For zero-order reactions, the rate constant has units of concentration per time (e.g., M/s). For first-order reactions, it’s expressed as reciprocal time (e.g., 1/s). Second-order reactions involve two reactants, so the rate constant is in units of volume per concentration per time (e.g., L/(mol·s)).
Units of Rate Constants: A Tale of Orders and Measurements
In the realm of chemical reactions, understanding the factors that govern their rates is crucial. Reaction order plays a pivotal role in this realm, and so do the units of rate constants. Just as a chef measures ingredients with different units (grams, milliliters, etc.), rate constants come in different units depending on the reaction order.
For zero-order reactions, the rate is independent of the concentration of reactants. The rate constant has units of concentration per unit time, such as moles per liter per second (mol/L/s). This unit reflects the fact that the rate is constant regardless of how much reactant is present.
First-order reactions, on the other hand, involve a single reactant whose concentration directly affects the rate. The rate constant for a first-order reaction has units of time inverse (1/s). The higher the rate constant, the faster the reaction proceeds for a given reactant concentration.
Finally, second-order reactions involve two reactants whose concentrations influence the rate. The rate constant for a second-order reaction has units of concentration inverse per unit time (1/L/s). This unit captures the dependency of the rate on both reactant concentrations, indicating that the reaction proceeds faster as the concentrations of both reactants increase.
These units are essential for quantifying the reaction rates, which plays a crucial role in various chemical processes, from pharmaceutical development to industrial manufacturing. By understanding the units of rate constants, scientists can accurately predict and control the rates of reactions, ensuring efficient and optimal outcomes.
Understanding the Arrhenius Equation: A Journey into Chemical Kinetics
Imagine you’re a chef preparing a delectable dish. The rate at which your masterpiece comes together depends on several factors, including the temperature of the oven, the activation energy needed to initiate reactions, and the concentration of ingredients.
Similarly, in the realm of chemistry, the rate of a reaction is influenced by a multitude of parameters. One fundamental concept in chemical kinetics is the Arrhenius equation, a powerful tool that helps us understand the interplay between these variables.
The Arrhenius equation states that the rate constant, k, of a reaction is directly proportional to the exponential of the negative activation energy, Ea, divided by the temperature, T, in Kelvin. Mathematically, it looks like this:
k = Ae^(-Ea/RT)
where A is the pre-exponential factor or frequency factor.
The activation energy represents the minimum amount of energy required for the reaction to occur, like the energy barrier that must be overcome for a chef to start cooking. A higher activation energy means a slower reaction rate, while a lower activation energy means a faster rate.
The temperature, on the other hand, represents the kinetic energy of the molecules. As the temperature increases, the molecules collide more frequently and with greater force, making it easier for them to overcome the activation energy barrier and react.
The pre-exponential factor, A, is a constant that reflects the frequency of collisions and the orientation of the molecules involved in the reaction.
By understanding the Arrhenius equation, we can delve into the intricacies of chemical reactions. It allows us to predict how the rate of a reaction will change with temperature, identify the activation energy of a specific reaction, and even explore the effects of catalysts on reaction rates.
So, next time you’re amazed by the speed at which your favorite dish is prepared, remember the Arrhenius equation – a testament to the fascinating relationship between temperature, activation energy, and the rate of chemical reactions.
Delving into Reaction Kinetics: Unraveling the Mysteries of Reaction Rates
Understanding Reaction Orders: The Blueprint of Reaction Speed
Every chemical reaction happens at a specific pace, governed by its reaction order. Zero-order reactions chug along at a constant rate, unaffected by reactant concentrations. First-order reactions accelerate linearly with reactant concentration, while second-order reactions zoom ahead as reactant concentrations multiply.
Integrated Rate Laws: The Equation that Tells the Tale
Integrated rate laws are the tales that reveal how reactant concentrations change over time. They help us determine reaction orders and calculate the rate constant, a key player in gauging the pace of a reaction.
Half-Life: The Point of No Return
Half-life is the time it takes for half of the reactants to disappear. It’s a trusty sidekick of the rate constant and reaction order, providing insights into their intricate dance.
Rate Constant: The Pulse of the Reaction
The rate constant is the heartbeat of a reaction. It measures the reaction speed and serves as a yardstick to compare different reactions.
Units of Rate Constant: The Language of Reaction Speed
Rate constants speak in different tongues, depending on the reaction order. Their units change, reflecting the number of reactants involved.
Arrhenius Equation: The Gateway to Activation Energy
The Arrhenius equation is a portal to understanding activation energy, the energy barrier that molecules must overcome to react. It unlocks the secrets of temperature and rate constant, revealing their intertwined symphony.
Activation Energy: The Hurdle to Reaction
Activation energy is the obstacle that reactants must leap over to ignite a reaction. Reactions with higher activation energies proceed more sluggishly, while those with lower energies race ahead.
Temperature Dependence of Rate Constant: A Dance of Heat and Speed
Temperature and rate constant Tango hand in hand. Higher temperatures boost the number of molecules with enough energy to overcome activation energy, leading to a faster reaction. The Arrhenius equation captures this graceful dance, linking temperature and rate constant.
Reaction kinetics is the art of timekeeping in the world of chemical reactions. By delving into reaction orders, integrated rate laws, and rate constants, we unravel the secrets of reaction rates. The Arrhenius equation, a beacon of knowledge, illuminates the path to understanding activation energy and its influence on reaction speed. Through this journey, we gain insights into the intricate dynamics that govern the chemical transformations that shape our world.
Understanding the Relationship Between Temperature and Rate Constant
In the realm of chemical reactions, temperature plays a crucial role in determining how quickly a reaction proceeds. The rate constant is a measure of this speed, and it is directly influenced by temperature.
The Arrhenius equation provides a mathematical framework for understanding this relationship:
k = A * exp(-Ea / RT)
Where:
- k is the rate constant
- A is the pre-exponential factor
- Ea is the activation energy
- R is the gas constant
- T is the temperature in Kelvin
The activation energy (Ea) represents the minimum energy that reactants must possess in order to undergo a reaction. The higher the activation energy, the slower the reaction. Conversely, a lower activation energy results in a faster reaction.
As temperature increases, the fraction of reactant molecules possessing the necessary activation energy also increases. This leads to a corresponding increase in the rate constant. In other words, reactions proceed faster at higher temperatures.
The Arrhenius equation illustrates this relationship with an exponential function. As temperature rises, the exponential term (-Ea / RT) becomes smaller, resulting in a larger value for the rate constant. This exponential dependence highlights the profound impact of temperature on reaction rates.
By measuring the rate constant at different temperatures, scientists can determine the activation energy of a reaction. This information is essential for understanding the reaction mechanism and predicting its behavior under varying temperature conditions. It also plays a crucial role in designing chemical processes, ensuring optimal reaction rates and yields.