Mastering Final Concentration: A Comprehensive Guide For Precise Calculations In Science
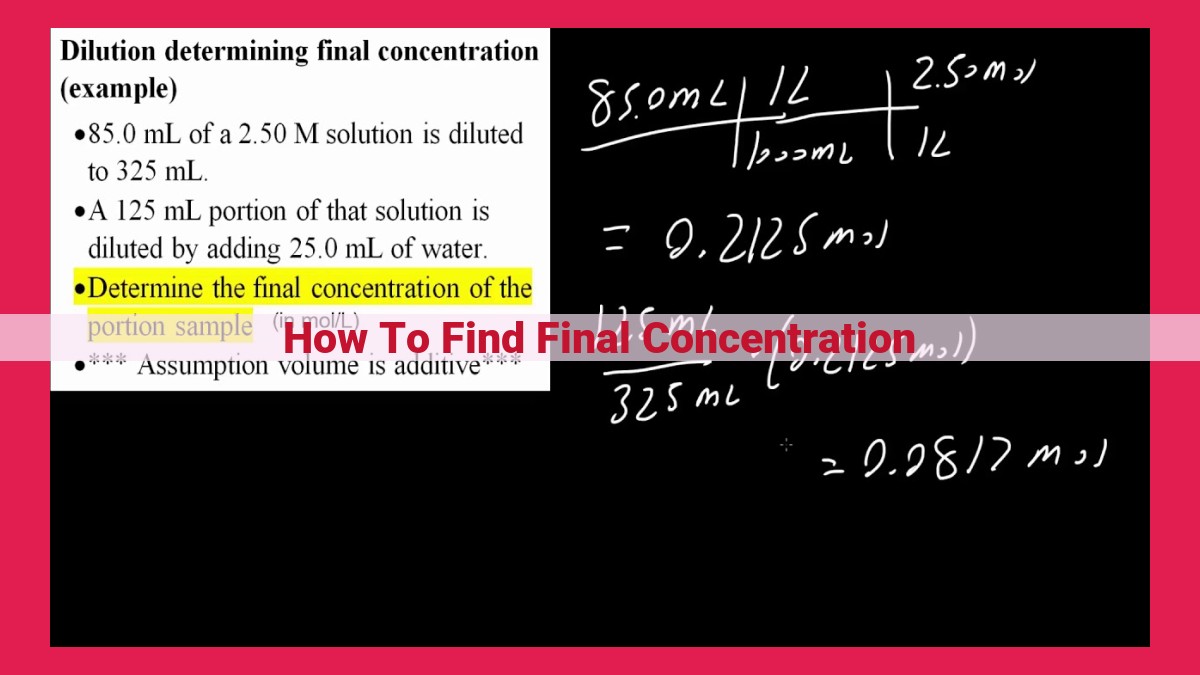
Determining final concentration is crucial in chemistry, biology, and medicine. The formula for finding final concentration is: Final Concentration = (Initial Concentration × Initial Volume) / Final Volume. This formula reflects the conservation of mass principle, where the amount of solute remains constant during dilution or concentration. To apply the formula, multiply the initial concentration by the initial volume and divide by the final volume. Factors like temperature and solvent effects can influence final concentration, so it’s essential to consider them. Understanding final concentration is essential for accurate experiments, drug administration, and various scientific endeavors, enabling researchers and practitioners to optimize solutions and achieve desired outcomes.
Final Concentration: A Crucial Consideration in Diverse Fields
In the realms of science, medicine, and industry, the concept of final concentration holds immense significance. It’s paramount to comprehend the significance of determining final concentration for various applications, from drug preparation in medical settings to chemical formulations in manufacturing processes.
Understanding final concentration enables precise control over the composition of solutions, ensuring their effectiveness and safety. In biological research, for instance, the final concentration of reagents used in experiments can influence the growth, viability, and behavior of cells. Similarly, in industrial settings, the final concentration of chemical reactants affects the yield and quality of products.
Grasping the Key Concepts
Before delving into the formula for calculating final concentration, it’s imperative to establish a solid understanding of the key concepts involved:
-
Initial Concentration: This represents the concentration of the solution before dilution. It’s typically expressed in units of moles per liter (M) or grams per liter (g/L).
-
Final Concentration: This pertains to the concentration of the solution after dilution. It’s likewise expressed in moles per liter (M) or grams per liter (g/L).
-
Volume of Initial Solution: This refers to the volume of the initial solution being diluted. It’s typically measured in liters (L) or milliliters (mL).
-
Volume of Final Solution: This represents the total volume of the diluted solution. It’s also measured in liters (L) or milliliters (mL).
-
Dilution Factor: This is a dimensionless quantity that represents the fold dilution of the initial solution. It’s calculated by dividing the final volume by the initial volume.
Key Concepts
- Initial Concentration: Explain its definition and units of measurement.
- Final Concentration: Define it and discuss its importance.
- Volume of Initial Solution: Describe its role and measurement units.
- Volume of Final Solution: Explain its impact on concentration.
- Dilution Factor: Define it and show how it relates to dilution.
Key Concepts
Understanding the concepts of initial concentration, final concentration, volume of initial solution, volume of final solution, and dilution factor is crucial for accurately determining the final concentration of a solution.
Initial Concentration
The initial concentration refers to the concentration of a solution before any dilution or addition of solvent. It is typically expressed in units of moles per liter (M) or millimoles per liter (mM). Understanding the initial concentration is essential for calculating the amount of solute that needs to be added or removed to achieve the desired final concentration.
Final Concentration
The final concentration is the concentration of a solution after dilution or addition of solvent. It is directly proportional to the initial concentration and inversely proportional to the final volume. The final concentration is a critical parameter in many scientific and industrial applications, as it determines the effectiveness and specificity of the solution.
Volume of Initial Solution
The volume of the initial solution refers to the volume of the solution before dilution or addition of solvent. It is typically measured in units of liters (L) or milliliters (mL). The volume of the initial solution is used in the formula for calculating the final concentration.
Volume of Final Solution
The volume of the final solution refers to the total volume of the solution after dilution or addition of solvent. It is typically larger than the volume of the initial solution. The volume of the final solution is inversely proportional to the final concentration, meaning that a larger final volume will result in a lower final concentration.
Dilution Factor
The dilution factor is a number that represents the ratio of the final volume to the initial volume. It is used to calculate the final concentration. A higher dilution factor indicates a greater dilution, resulting in a lower final concentration.
Unveiling the Formula for Final Concentration: A Journey of Dilution and Concentration
In the realm of science and beyond, final concentration plays a pivotal role. From preparing solutions in the laboratory to understanding the dynamics of chemical reactions and biological processes, determining the final concentration is crucial. This formula encapsulates the essence of dilution, the process of altering a solution’s concentration by adding solvent.
At the heart of this formula lies the principle of conservation of mass: the total amount of solute remains constant throughout the dilution process. This principle guides us as we manipulate the initial concentration, initial volume, and final volume to achieve the desired final concentration.
The formula for calculating final concentration is:
**Final Concentration = (Initial Concentration × Initial Volume) / Final Volume**
Let’s break down each term:
- Initial Concentration: The concentration of the solution before dilution, typically expressed in units of moles per liter (M).
- Initial Volume: The volume of the initial solution, usually measured in milliliters (mL) or liters (L).
- Final Volume: The volume of the diluted solution, including both the initial solution and the added solvent, also measured in mL or L.
By understanding and applying this formula, you gain the power to tailor solutions to specific concentrations, ensuring accuracy and precision in your experiments and applications. Join us as we delve into the practical aspects of using this formula, empowering you with the knowledge to navigate the world of concentrations with confidence.
Applying the Formula: Unraveling the Concentration Mystery
Now, let’s get our hands dirty and apply the formula to solve real-world concentration problems.
- Step 1: Gather Your Ingredients
You’ll need three essential ingredients: initial concentration, initial volume, and final volume. Make sure you have accurate measurements for each.
- Step 2: The Magic Formula
Plug your ingredients into the magical formula:
Final Concentration = (Initial Concentration × Initial Volume) / Final Volume
- Step 3: An Illustrious Example
Let’s say you have 50 mL of a 1 M salt solution and want to dilute it to a final volume of 100 mL. Using our formula:
Final Concentration = (1 M × 50 mL) / 100 mL = 0.5 M
Voila! Your new solution is 0.5 M.
Pro Tip: It’s like baking a cake. If you double the final volume, you halved the final concentration.
Additional Considerations: The Hidden Factors
When dealing with concentrations, there are a few sneaky factors that can influence the outcome:
- Temperature: Temperature changes can alter the volume of solutions, affecting the final concentration.
- Solvent Effects: Different solvents can interact with solutes, affecting their behavior and concentration.
Remember: Understanding these factors helps you navigate the complexities of concentration calculations with precision.
Additional Considerations: Factors That Can Influence Final Concentration
While the formula for final concentration is straightforward, several factors can influence the accuracy and reliability of your calculations. These factors include:
Temperature: Temperature changes can affect the volume of both the initial and final solutions, leading to variations in final concentration. As temperature increases, solutions generally expand, resulting in a lower final concentration if the volume is not adjusted accordingly.
Solvent Effects: Not all solvents behave identically. Some solvents can interact with the solute or undergo chemical reactions that alter the concentration. For example, using water as a solvent for an acid-base reaction can lead to ionization and changes in concentration compared to using a non-polar solvent.
Glassware and Volumetric Errors: The accuracy of pipettes, burettes, and volumetric flasks can impact final concentration. Calibrated glassware and careful measuring techniques are crucial to minimize volumetric errors and ensure precise results.
It’s important to note that these factors can affect the final concentration to varying degrees depending on the specific system being studied. Researchers should consider these factors and take appropriate measures to control them when determining final concentrations.