Mastering Equivalence Point Detection In Titration: A Comprehensive Guide
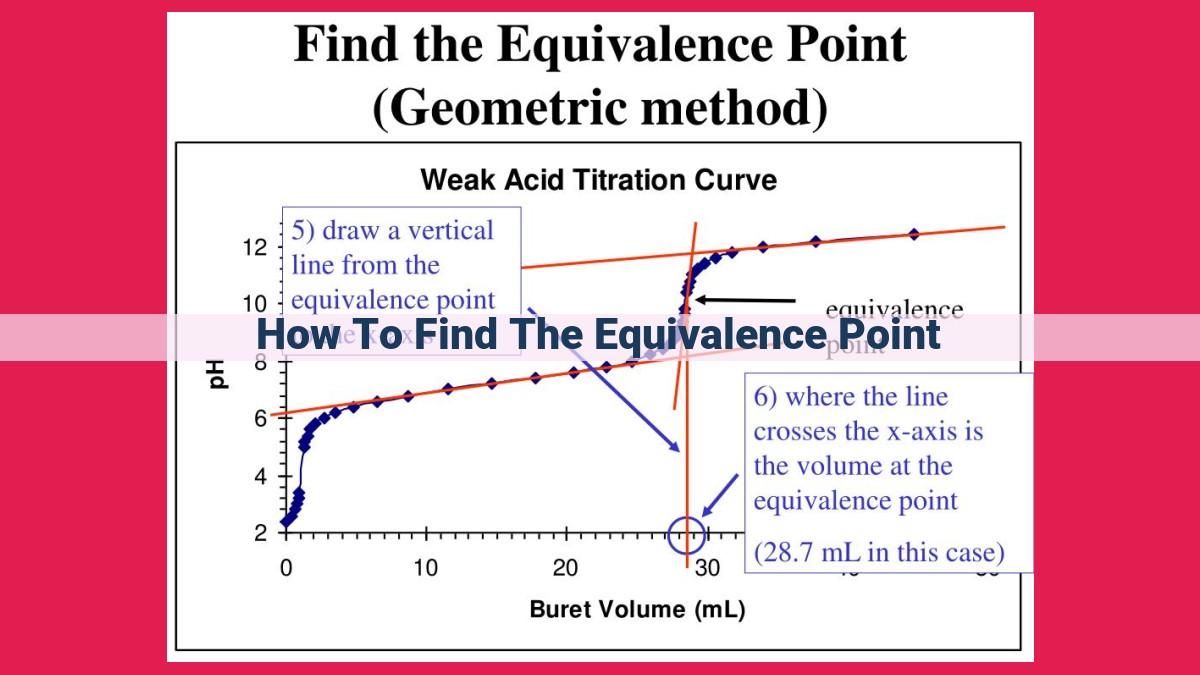
To find the equivalence point in titration, observe the change in pH and indicator color as the titrant is added to the analyte. The equivalence point occurs when the moles of titrant added are equal to the moles of analyte present, resulting in complete neutralization. For acid-base titrations, the equivalence point is indicated by a pH of 7 if using a neutral indicator like phenolphthalein, which turns colorless at the equivalence point.
Definition of titration and its purpose
Understanding Titration: Unlocking the Secrets of Chemistry
Title of Page: Titration: A Comprehensive Guide for Chemistry Enthusiasts
Meta Description: This comprehensive guide on titration will guide you through the basics, from understanding its purpose and concepts to mastering techniques using indicators, graduated cylinders, and burettes.
I. Titration: The Essence of Chemistry
Titration is the technique in chemistry that lets you precisely measure the concentration of a known substance by reacting it with a carefully measured amount of another substance with a known concentration.
Through this chemical dance, you can determine the exact point where these two substances perfectly balance each other, revealing crucial information about the unknown substance’s concentration. And this, my friend, is the equivalence point – a pivotal moment in titration.
Sub-Heading: Key Concepts
Before diving deeper, let’s get acquainted with some essential terms:
- Equivalence point: The magical moment when the moles of acid and base are equal.
- Neutralization reaction: The chemical tango between an acid and a base, resulting in a neutral solution.
- Indicator: A color-changing substance that tells you when the equivalence point is near.
- Graduated cylinder: Your trusty tool for precisely measuring volumes.
- Burette: The star of the show, allowing you to disperse liquids with unmatched accuracy.
Overview of related concepts: equivalence point, neutralization reaction, indicator, graduated cylinder, burette
Embark on a captivating journey into the world of titration, a fundamental technique used by scientists to unravel the secrets of chemical reactions. Titration, the precise process of adding one solution to another until they react completely, is a key tool in a chemist’s arsenal, shedding light on the composition and properties of substances.
In this comprehensive exploration, we’ll navigate the intricacies of titration, unraveling its components and emphasizing its significance. Begin by delving into the concept of equivalence point, the crucial moment when reactants are fully consumed, leaving no trace behind. Learn to identify this point with ease, unraveling the mysteries of neutralization reactions.
Unveiling Neutralization Reactions
Neutralization reactions, a dance between acids and bases, stand as the heart of titration. These reactions, marked by the formation of water and a salt, provide a gateway to understanding the strength of acids and bases. Delve into their equation and characteristics, unlocking the secrets of their role in the titration process.
pH: The Chemical Compass
pH, a measure of the acidity or alkalinity of a solution, acts as a compass in the realm of titration. Its correlation with the equivalence point guides us towards the completion of chemical reactions. Discover the concept of pH, grasping its significance in identifying the turning point of titration.
Phenolphthalein: The Colorful Guide
Phenolphthalein, a versatile indicator, plays a pivotal role in titration, signaling the arrival of the equivalence point with its vibrant color change. Unravel its properties and mechanism, understanding its advantages and limitations, ensuring accurate results in your titrations.
Indicators: The Sentinels of Reactions
Indicators, the gatekeepers of titration, serve as the eyes of the chemist, alerting to the completion of reactions. Explore their definition and function, unraveling their role in signaling the endpoint. Discover the diverse types of indicators, each playing a unique part in the titration dance.
Graduated Cylinder: Measuring with Precision
Graduated cylinders, the stalwarts of volume measurement, take center stage in titration. Master the art of accurate volume measurement, ensuring the integrity of your results. Learn the techniques and nuances of using graduated cylinders, recognizing their importance in precise titration.
Burette: Dispensing with Finesse
Burettes, the precision instruments of titration, reign supreme in dispensing precise volumes of liquids. Delve into their design and calibration, understanding their meticulous maintenance for accurate liquid delivery. Uncover the techniques for using burettes in titration, achieving the finesse required for reliable results.
The Equivalence Point: Unraveling the Heart of Titration
In the realm of chemistry, titration stands as a sophisticated technique to precisely determine the concentration of an unknown solution. At its core lies the equivalence point, a pivotal juncture where the reactants in a chemical reaction combine in exactly the stoichiometric ratio.
The equivalence point holds immense significance for several reasons. First and foremost, it marks the completion of the chemical reaction. Imagine a dance where two reactants, like Romeo and Juliet, gracefully enter the stage. As the reaction progresses, they increasingly come together, until at the equivalence point, they are perfectly paired, symbolizing the full consumption of both reactants.
Furthermore, the equivalence point provides a precise indication of the unknown solution’s concentration. By determining the exact volume of titrant (the solution with known concentration) required to reach the equivalence point, chemists can calculate the concentration of the unknown solution with astonishing accuracy.
Recognizing the equivalence point is a skill that requires careful observation and a keen understanding of chemical reactions. For example, in acid-base titrations, the equivalence point is often marked by a sudden color change of the indicator, a special chemical that senses the completion of the reaction. By attentively observing these subtle clues, chemists can pinpoint the elusive equivalence point.
Understanding Titration: A Journey to Discover the Equivalence Point
Recognizing the Tipping Point
In the realm of chemistry, titration reigns supreme as a technique for determining the exact concentration of an unknown solution. At the heart of this intricate process lies the equivalence point, the moment when the solution being analyzed and the reagent being added to it reach perfect stoichiometric balance.
Pinpointing the equivalence point is a crucial step in any titration, as it marks the completion of the reaction and allows for precise calculations. Just like a detective unraveling a mystery, we, as chemists, use various clues to decipher the moment of equilibrium.
One telltale sign is a color change in the solution. Indicators, substances that change color at specific pH values, act as our chemical detectives. When the indicator detects the correct pH corresponding to the equivalence point, it signals the transformation, revealing the moment of truth.
Another clue involves pH measurements. pH, a measure of acidity or basicity, undergoes a dramatic shift as the titration progresses. Closely monitoring the pH allows us to pinpoint the equivalence point where pH reaches a specific value.
Finally, physical observations can also provide insights into the equivalence point. For example, in certain titrations, the formation of a precipitate, a solid that forms during the reaction, can indicate the end point.
By carefully observing these clues, chemists can determine the equivalence point with precision, unlocking the secrets of unknown solutions and paving the way for accurate analysis.
Importance of pH in Determining the Equivalence Point
In titration, the equivalence point is the crucial moment when the reactants have completely reacted with each other. Determining the equivalence point accurately is essential to obtain reliable results. Here’s where pH plays a pivotal role.
pH, a measure of the acidity or basicity of a solution, provides valuable insights into the progress of the titration. During a neutralization titration, where an acid and a base react to form salt and water, the pH changes gradually as the reactants approach the equivalence point.
As the titration proceeds, the pH gradually shifts towards neutrality. When the equivalence point is reached, the solution becomes exactly neutral, with a pH of 7. This is because the acid and base have neutralized each other completely, forming a solution that contains equal concentrations of H+ and OH- ions.
It’s important to note that the pH change during a titration can vary depending on the strength of the acid and base being used. For strong acids and bases, the pH change is rapid and more distinct, while for weak acids and bases, it’s more gradual.
Understanding the correlation between pH and the equivalence point is crucial in titration. It allows chemists to determine the equivalence point more precisely, especially when using visual indicators, which change color at specific pH values. By monitoring the pH throughout the titration, chemists can visually identify the point at which the solution becomes neutral, thus signaling the equivalence point.
In conclusion, pH plays a vital role in determining the equivalence point in titration. By understanding the pH changes that occur during the titration, chemists can obtain accurate and reliable results.
Titration: Unraveling the Secrets of Chemical Reactions
Embark on a scientific adventure as we dive into the fascinating world of titration. It’s a technique used by chemists to unveil the mysteries of chemical reactions, and it’s like a dance between two solutions.
One solution, the analyte, contains an unknown concentration of a substance. The other, the titrant, has a known concentration. By carefully adding the titrant to the analyte, we reach a special point called the equivalence point.
The equivalence point is the moment of perfect balance, where the number of moles of acid equals the number of moles of base. It’s like a chemical handshake, where the two solutions reach a harmonious equilibrium.
At this point, the reaction between the acid and base is complete, and we have a neutralization reaction. It’s like two opposing forces coming together to create something new. These reactions create salts, which are ionic compounds that form when an acid and a base react.
The beauty of neutralization reactions is that they can be used to determine the exact concentration of an unknown solution. By carefully observing the reaction, we can use the principles of titration to unlock the secrets of chemical mysteries.
III. Neutralization Reaction
A Tale of Two Reactants: Acids and Bases
In the realm of chemistry, a neutralization reaction is a magical dance between two opposing forces: acids and bases. Acid, like a grumpy wizard, possesses an excess of hydrogen ions (H+). Base, on the other hand, embodies the spirit of a generous benefactor, carrying a surplus of hydroxide ions (OH-).
The Dance of Neutralization
When these two chemical opposites meet, they engage in a transformative dance. Hydrogen ions and hydroxide ions gracefully pair up, forming water (H2O)—a symbol of harmony and balance. This elegant reaction neutralizes the acidic and basic properties, resulting in a solution that is neither acidic nor basic but rather neutral.
Characteristics of Neutralization Reactions
The equation for a neutralization reaction is as follows:
Acid + Base → Salt + Water
Key characteristics of neutralization reactions include:
- Exothermic: They release heat, making the solution feel warm.
- Complete: Acids and bases react completely, leaving no unreacted reactants behind.
- Irreversible: Once the reaction occurs, it cannot be reversed.
- Formation of Salt: The reaction produces a salt, which is a compound made up of positively charged ions (cations) from the base and negatively charged ions (anions) from the acid.
Role of Neutralization Reactions in Titrations
In the realm of chemistry, titrations stand as a cornerstone technique for determining the precise concentration of an unknown substance. At the heart of this process lies the neutralization reaction, a chemical dance between acids and bases that plays a pivotal role in achieving accurate results.
When an acid and base are combined, they undergo a chemical reaction that forms salt and water. This reaction is characterized by the transfer of hydrogen ions from the acid to the base, resulting in a neutral solution. In the context of titrations, we exploit this reaction to determine the exact amount of acid or base present in a sample.
In a typical titration, a known volume of an acid or base of known concentration (the titrant) is gradually added to a sample of the unknown solution. As the titrant is added, it reacts with the unknown substance, and the pH of the solution changes. The equivalence point is reached when the moles of acid and base are equal, and the solution becomes exactly neutral. At this point, the reaction is complete, and the volume of titrant used can be used to calculate the concentration of the unknown substance.
The strength of the acid and base involved influences the pH at the equivalence point. Strong acids and bases react completely to form a neutral solution with a pH of 7, while weak acids and bases produce a solution with a pH that is slightly acidic or basic.
Understanding the role of neutralization reactions in titrations is crucial for performing accurate and reliable experiments. By carefully observing the color change of an indicator, we can pinpoint the equivalence point and determine the exact concentration of the unknown substance in the sample.
Concept of pH and its Measurement
Imagine if we had a magic wand that could tell us how “acidic” or “basic” a solution is. Well, that wand is called pH. It’s a measure that ranges from 0 to 14, where:
- 0 to 6: Acidic solutions (with 0 being the most acidic)
- 7: Neutral solutions
- 8 to 14: Basic or alkaline solutions (with 14 being the most basic)
Measuring pH accurately is crucial for various scientific disciplines, from chemistry and biology to environmental monitoring. One of the most reliable ways is using a pH meter, an instrument that measures the electrical potential between a glass electrode and a reference electrode placed in the solution.
However, for simplicity and cost-effectiveness, we often use indicators. These are substances that change color at specific pH ranges. For example, litmus paper turns red in acidic solutions and blue in basic solutions. Phenolphthalein indicator is another popular choice, changing from colorless in acidic solutions to pink in basic solutions.
By observing the color change of the indicator, we can estimate the pH of the solution. This is especially useful in titrations, where we gradually add one solution to another until a certain reaction is complete. By monitoring the pH using an indicator, we know exactly when to stop adding the solution, ensuring accurate results.
Remember, understanding pH and how to measure it is not just a chemistry lesson but a valuable tool in various fields, helping us make informed decisions about the solutions around us.
The Intriguing Connection Between pH and Equivalence Point in Titrations
In the realm of chemistry, titrations are like detective work, where we carefully add one solution to another until a mysterious reaction reaches its climax. This point of maximum intrigue is known as the equivalence point, where chemical balance is achieved. But how do we know when we’ve reached this critical juncture? Enter pH, a measure of acidity or basicity, which plays a pivotal role in unraveling this mystery.
Different Types of Titrations, United by pH
Titrations come in various flavors, depending on the nature of the reactants. Acid-base titrations, the most common type, involve neutralizing an acid with a base, or vice versa. Redox titrations delve into oxidation-reduction reactions, while precipitation titrations focus on the formation of insoluble solids. Yet, amidst this chemical diversity, pH remains a unifying force.
In acid-base titrations, the equivalence point corresponds to the point where the number of moles of acid equals the number of moles of base. This perfect balance creates a neutral solution, where the pH is exactly 7. However, for strong acid and strong base titrations, the pH change is relatively gradual, making it trickier to pinpoint the exact equivalence point.
Conversely, in weak acid and weak base titrations, the pH change is more pronounced, providing a clearer signal of the equivalence point. This is because the dissociation of weak acids and bases is incomplete, creating a buffer solution that resists significant pH changes until the equivalence point is nearly reached.
pH: The Ultimate Detective
So, how does pH guide us to the equivalence point? Indicators, special substances that change color at a specific pH, act as our chemical detectives. When the pH reaches the equivalence point, the indicator undergoes a color change, revealing the hidden truth about the reaction’s progress.
Phenolphthalein, a common indicator in acid-base titrations, changes from colorless to pink at a pH around 8.2–10.0. This means that in titrating a strong acid with a strong base, phenolphthalein will turn pink close to the equivalence point.
Methyl orange, on the other hand, changes from red to yellow at a pH around 3.1–4.4. It is often used in titrating a weak acid with a strong base, signaling the equivalence point when the solution turns yellow.
By understanding the correlation between pH and equivalence point, we unlock the secrets of successful titrations. It’s like having a built-in GPS, guiding us through the chemical maze with precision and elegance.
How to determine the pH at the equivalence point
Understanding Titration: A Step-by-Step Guide
What is Titration?
Titration is a laboratory technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. This process involves gradually adding the known solution to the unknown until the reaction reaches its endpoint, known as the equivalence point.
The Equivalence Point: A Turning Point
The equivalence point marks the moment when the moles of reactants are equal. It’s like a chemical dance where the two solutions find perfect balance. Recognizing the equivalence point is crucial, as it’s the key to calculating the concentration of the unknown solution.
Neutralization Reactions: The Chemistry Behind Titration
Many titrations involve neutralization reactions, where an acid and base react to form salt and water. The equivalence point in these reactions occurs when the acid and base are completely neutralized, resulting in a neutral solution.
pH and the Equivalence Point in Different Titrations
pH measures the acidity or basicity of a solution. In strong acid-strong base titrations, the pH at the equivalence point is 7, indicating a neutral solution. However, in weak acid-weak base titrations, the pH at the equivalence point deviates from 7.
How to Determine the pH at the Equivalence Point
To find the pH at the equivalence point, you need to know the ionization constants of the acid and base involved in the reaction. Using a mathematical calculation, you can determine the pH at the equivalence point. This value is crucial for calculating the exact concentration of the unknown solution.
Role of Indicators in Titration
Indicators are substances that change color at a specific pH. In titration, they signal the completion of the reaction by indicating the equivalence point. Phenolphthalein is a common indicator that turns pink at the equivalence point in an acid-base titration.
Graduated Cylinder: Volume Measurement
Graduated cylinders measure volumes accurately. In titration, they are used to measure the initial volume of the unknown solution. Precise volume measurement is essential for obtaining accurate results.
Burette: Dispensing Precision
A burette is a precise instrument used to dispense a known volume of the known solution. It’s calibrated to deliver volumes with high accuracy. In titration, the burette is used to add the known solution to the unknown solution, allowing for gradual titration and precise determination of the equivalence point.
Understanding Phenolphthalein: The Color-Changing Indicator
Introduction:
In the realm of chemistry, where precision and accuracy reign supreme, indicators play a crucial role in unveiling the mysteries of chemical reactions. Among these indicators, phenolphthalein stands out as a versatile and fascinating tool, captivating the minds of scientists for centuries.
Mechanism of Phenolphthalein:
Phenolphthalein is a colorless compound that undergoes a remarkable transformation when exposed to certain pH conditions. As the pH increases, its structure rearranges, causing a profound shift in its appearance.
At low pH, phenolphthalein exists as a colorless molecule, harmoniously blending with its surroundings. However, as the pH rises, it metamorphosizes into a vibrant pink shade, signaling a fundamental change in the chemical environment.
Color Change at the Equivalence Point:
During titration, a specialized technique used to determine unknown concentrations, phenolphthalein finds its true calling. It acts as a sentinel, alerting us to the precise moment when two solutions have perfectly neutralized each other, known as the equivalence point.
As the titrant (the solution of known concentration) is gradually added to the analyte (the solution of unknown concentration), phenolphthalein remains inert. But as the equivalence point draws near, the pH begins to climb, triggering the dramatic color change from colorless to pink.
Advantages and Limitations:
Phenolphthalein’s virtues include its sharp color change and suitability for titrating weak acids with strong bases. However, its limitations must also be acknowledged.
Phenolphthalein is not suitable for titrating strong acids as the color change occurs before the equivalence point. Additionally, some substances may interfere with its color change, potentially leading to inaccuracies.
Conclusion:
Phenolphthalein, a captivating chemical with a rich history, continues to be an indispensable tool in the analytical chemist’s arsenal. Its ability to signal the equivalence point with precision and clarity makes it a trusted companion in the pursuit of chemical knowledge.
Color Change of Phenolphthalein at the Equivalence Point
In the enchanting world of titration, a remarkable transformation occurs when phenolphthalein, a colorless indicator, witnesses the delicate dance between acids and bases. As the burette steadily dispenses the titrant, the graduated cylinder filled with the unknown solution quietly reacts.
Suddenly, at the equivalence point – the moment of perfect balance – a dramatic metamorphosis takes place. The colorless phenolphthalein springs to life, blushing a vibrant pink. This remarkable color change is a symphony of chemistry, signaling that the acid and base have neutralized each other completely.
The equivalence point, a pivotal moment in titration, marks the exact moment when the moles of acid equal the moles of base. It’s a harmonious equilibrium where the acidic and basic properties cancel each other out, creating a neutral solution.
The color change of phenolphthalein is not just a visual cue; it is a testament to the intricate chemical reactions taking place. As the last drop of titrant is added, the pH of the solution shifts above 8.2, indicating a basic environment. This change in pH triggers the phenolphthalein molecules to undergo a structural transformation, resulting in the brilliant pink hue.
The choice of phenolphthalein as an indicator for acid-base titrations is not arbitrary. Its color change is sharp and distinct, making it easy to identify the equivalence point accurately. Additionally, it is reversible, meaning its color can revert back to colorless if an excess of acid is added.
Understanding the color change of phenolphthalein at the equivalence point is crucial in titration. It allows chemists to determine the exact concentration of unknown solutions, a fundamental skill in countless industries, from environmental monitoring to pharmaceutical research. It’s a testament to the captivating interplay between chemistry and color, a reminder that even the smallest changes can unveil hidden truths.
Phenolphthalein: A Vital Indicator in Titrations
In the realm of chemistry, titration reigns supreme as a technique for determining the concentration of an unknown solution. Among the crucial components of titration is the indicator, a substance that signals the completion of the reaction by undergoing a noticeable color change. One such indicator, phenolphthalein, plays a pivotal role in titrations, offering both advantages and limitations.
Properties and Mechanism
Phenolphthalein is a weak acid that exists in two forms: colorless in acidic solutions and bright pink in basic solutions. Its color change occurs around pH 8.2-10.0. This property makes it particularly suitable for use in titrations involving strong acids and strong bases, where the equivalence point (the point at which the acid and base are completely neutralized) falls within this pH range.
Advantages
- Sharp Color Change: Phenolphthalein exhibits a sharp and distinct color change, making it easy to identify the equivalence point accurately.
- Wide pH Range: It has a relatively wide pH range of transition, ensuring its effectiveness in various types of titrations.
- Inexpensive and Readily Available: Phenolphthalein is a cost-effective and accessible indicator, making it widely used in educational and analytical settings.
Limitations
- pH Sensitivity: Phenolphthalein is sensitive to pH, and its color change can be influenced by slight variations in pH. This can lead to errors in titrations involving weak acids or bases.
- Not Suitable for Colored Solutions: In colored solutions, the color change of phenolphthalein may be difficult to observe, limiting its applicability in certain situations.
- Incompatible with Strong Oxidizing Agents: Phenolphthalein can be oxidized by strong oxidizing agents, such as potassium permanganate. This can result in a permanent color change, affecting the accuracy of the titration.
Phenolphthalein remains a valuable indicator in titrations, especially for reactions involving strong acids and bases. While its advantages, such as sharp color change and wide pH range, make it a reliable choice, it is essential to consider its limitations when selecting an indicator for a particular titration. By understanding these advantages and limitations, chemists can harness the power of phenolphthalein to obtain accurate and precise results in their titrations.
Definition and function of indicators in titration
Understanding Indicators: The Essential Guide to Titration Magic
In the realm of chemistry, titration stands out as a technique that allows scientists to uncover the secrets of unknown substances. At the heart of this process lies a special ingredient known as an indicator. Like a wizard’s wand, indicators play a crucial role in signaling the completion of the titration reaction, revealing the mystery hidden within.
What is Titration?
Titration is a chemical technique used to determine the concentration of an unknown substance. By carefully adding a known solution (titrant) to the unknown solution (analyte), we can reach a point known as the equivalence point. It’s like finding the perfect balance on a scale, where neither solution has the upper hand.
The Role of Indicators
Indicators are substances that undergo a color change at or near the equivalence point. They act as the “eyes” of the titration, providing a visual cue to the experimenter. The color change signals that the reaction is complete and the desired information about the unknown substance has been unlocked.
How Indicators Work
Indicators are added to the analyte solution before the titration begins. As the titrant is added, the indicator molecules interact with the chemical species in the solution. When the solution reaches the equivalence point, the indicator molecules experience a structural change, causing their color to change. This color change is what alerts the experimenter that the titration is complete.
Types of Indicators
Different indicators are used for different types of titrations. Phenolphthalein, for example, is a common indicator used in acid-base titrations. It turns colorless in acidic solutions and pink in basic solutions. This means that it will change color when the equivalence point is reached in an acid-base titration.
Importance of Accurate Volume Measurements
Precise volume measurements are essential in titration. Graduated cylinders are used to measure the volume of the titrant, while burettes are used to dispense the titrant in small, controlled amounts. Accurate volume measurements ensure that the titration results are reliable and can be used to calculate the unknown concentration accurately.
Titration: A Chemical Balancing Act
Imagine yourself as a culinary chemist, carefully balancing ingredients to create a delectable concoction. Just like in cooking, chemistry has its own balancing technique called titration, a process that determines the exact concentration of a substance in a solution. Let’s delve into the world of titration and unravel its fascinating steps.
The Equivalence Point: A Moment of Balance
During titration, we’re aiming for a precise moment known as the equivalence point, the point where the reactants are perfectly balanced, like harmonious dancers on a stage. To recognize this point, we rely on indicators, chemical substances that undergo a dramatic color change when the reaction reaches its endpoint.
Indicators: Signaling the Completion
Think of indicators as chemical messengers, waving a flag when the battle is won. They change color based on the acidity or alkalinity of the solution, alerting us that the reaction has reached its peak. For example, phenolphthalein, a popular indicator, turns a vibrant pink at the equivalence point, indicating that the solution has become neutral, like a perfectly balanced scale.
Neutralization Reactions: The Chemical Balancing Act
The dance between acids and bases, known as neutralization reactions, plays a crucial role in titration. These reactions produce a salt and water, leveling the playing field of acidity and alkalinity. During titration, by carefully adding one reactant to the other, we reach the equivalence point where the solution becomes neutral, like a diplomatic agreement between opposing forces.
Graduated Cylinders and Burettes: Precision Players
In the titration laboratory, precision is paramount. Graduated cylinders help us accurately measure the volume of liquids, like measuring cups for our chemical concoctions. For even greater precision, we use burettes, specialized tools that dispense liquids with remarkable accuracy. These tools ensure that we don’t overshoot or undershoot our target volume, keeping our titration dance on track.
Different types of indicators and their applications
Titration: A Guide to Understanding the Chemistry of Equivalence, Neutralization, and Indicators
I. Understanding Titration
Titration is a fundamental technique used in chemistry to determine the concentration of an unknown solution by reacting it with a solution of known concentration. This process involves gradually adding the known solution to the unknown solution until a specific chemical reaction, known as the neutralization reaction, is complete.
II. The Equivalence Point
The equivalence point marks the completion of the neutralization reaction, where the acid and base have reacted in stoichiometric proportions. This point is crucial because it indicates the exact moment when the number of moles of acid equals the number of moles of base.
III. Neutralization Reaction
A neutralization reaction is a chemical reaction between an acid and a base that produces a salt and water. This reaction plays a vital role in titrations, as it is the basis for determining the unknown concentration.
IV. pH and Equivalence Point
The pH of a solution measures its acidity or alkalinity. In titrations, the pH **changes dramatically around the equivalence point. Understanding the relationship between pH and the equivalence point helps ensure accurate results.
V. Phenolphthalein as an Indicator
Phenolphthalein is a common indicator used in titrations. It is a weak acid that changes color from colorless to pink at the equivalence point. Other indicators have different color changes, but all serve the same purpose of signaling the completion of the reaction.
VI. Role of Indicators in Titration
Indicators are substances that change color at specific pH values, indicating the endpoint of the titration. They play a crucial role in titrations, as they provide a visual cue of the equivalence point.
VII. Graduated Cylinder: Measuring Volume
Graduated cylinders are used to measure the volume of liquids in titrations. They are calibrated with precise markings to ensure accurate measurement.
VIII. Burette: Dispensing Precise Volume
Burettes are specialized glassware used to dispense precise volumes of liquids in titrations. They are calibrated to deliver accurate volumes, ensuring precise control of the solution added.
Titration is a versatile technique that allows scientists to determine the concentration of unknown solutions. Understanding the concepts of equivalence point, neutralization reaction, and indicators is essential for successful titration experiments. By following these principles, researchers can accurately analyze chemical reactions and obtain reliable results.
Understanding Titration: A Journey into Precision Chemistry
In the realm of chemistry, titration stands out as a technique that allows us to measure the concentration of a solution with remarkable accuracy. This blog post embarks on a journey to unravel the intricacies of titration, exploring its fundamental concepts and practical applications.
The Essence of Titration
Imagine a chemist seeking to determine the concentration of an unknown acid solution. Titration offers the solution to this puzzle. It involves gradually adding a known solution (called the titrant) of a base to the unknown acid solution, while carefully monitoring the reaction. This process continues until the equivalence point is reached.
The Equivalence Point: A Balancing Act
The equivalence point marks the moment when the moles of acid and base are equal. It’s like a chemical handshake where the reactants perfectly balance each other out. Recognizing the equivalence point is crucial as it indicates the completion of the reaction.
Neutralization Reaction: The Titration’s Heartbeat
The reaction at the core of titration is a neutralization reaction. This occurs when an acid and a base combine to form salt and water, releasing heat in the process. Neutralization reactions play a pivotal role in titration, as they allow us to calculate the concentration of the unknown solution based on the known concentration of the titrant.
pH and Equivalence Point: A Delicate Relationship
pH is a measure of the acidity or basicity of a solution. During titration, the pH changes as the reaction progresses. By monitoring the pH, we can estimate the equivalence point.
Phenolphthalein: The Indicator That Signals Completion
Indicators are substances that change color at specific pH values. In titration, phenolphthalein is commonly used as an indicator. It remains colorless in acidic solutions and turns pink when the equivalence point is reached, signaling the completion of the reaction.
Measuring with Precision: Graduated Cylinders and Burettes
Graduated cylinders and burettes are essential tools in titration. Graduated cylinders are used to measure volumes of liquids, while burettes are designed for dispensing precise volumes. Accurate measurements are vital for reliable titration results.
Titration is a powerful technique that enables chemists to precisely determine the concentration of solutions. Understanding the concepts of equivalence point, neutralization reaction, pH, indicators, graduated cylinders, and burettes is essential for performing successful titrations. With these tools at our disposal, we can venture into the fascinating world of chemistry, unraveling the mysteries of solutions one drop at a time.
Techniques for Accurate Volume Measurement Using Graduated Cylinders
In the realm of chemistry, precision is paramount, and when it comes to volume measurement, graduated cylinders are an indispensable tool. These cylindrical vessels, adorned with meticulously etched刻刻 marks, empower scientists to precisely quantify the volumes of liquids—a crucial aspect of many chemical experiments.
To ensure utmost accuracy, it’s imperative to employ the proper technique when using graduated cylinders. Begin by selecting the appropriate size cylinder for the volume you intend to measure. The cylinder should be large enough to accommodate the entire volume without overflowing, but not so large that the liquid level falls below the lowest刻刻 mark.
Next, position the cylinder on a level surface and ensure it is stable. Hold the cylinder vertically and bring the liquid to the desired level. The meniscus, the curved surface of the liquid, should be read at eye level. To achieve the most accurate reading, align your eye with the bottom of the meniscus.
Avoid parallax error by taking the reading directly perpendicular** to the graduated刻刻 marks. This ensures that you’re measuring the true liquid level and not an oblique angle. Be mindful of the temperature as it can affect the volume of the liquid. Always use graduated cylinders calibrated for the specific temperature at which you’re measuring.
Finally, record the volume precisely, noting the lowest graduation刻刻 mark that is completely submerged by the liquid. If the liquid level falls exactly on a graduation刻刻 mark, record that value. If it falls between刻刻 marks, estimate the volume to the nearest subdivision.
By adhering to these techniques, you can confidently measure volumes using graduated cylinders with remarkable accuracy, ensuring the validity of your experimental results.
The Importance of Precise Volume Measurements in Titration: Unraveling the Secrets of Acid-Base Reactions
Titration: A Delicate Dance of Precision
In the realm of chemistry, titration emerges as a meticulous technique that precisely unveils the unknown concentration of a solution. This analytical masterpiece involves cautiously adding a solution with a known concentration, known as the titrant, to another solution whose concentration is yet to be determined, known as the analyte.
The Endpoint: A Critical Turning Point
As the titrant is gradually dispensed into the analyte, the solution undergoes a series of subtle chemical transformations. The endpoint, a pivotal moment in the titration process, marks the point where the reaction between the titrant and the analyte is complete. This crucial juncture signifies that the amount of titrant added is stoichiometrically equivalent to the amount of analyte present.
Precise Measurements: The Key to Accurate Results
Achieving accurate titration results hinges on the meticulous measurement of both the titrant and analyte volumes. The graduated cylinder, a mainstay in any chemistry lab, serves as a reliable tool for measuring larger volumes of liquids. However, for applications demanding higher precision, the burette takes center stage.
The Burette: Precision Personified
The burette, with its meticulously calibrated scale and precision valve, empowers chemists to dispense precise volumes of titrant. Precise volume delivery is paramount because even a slight deviation can lead to inaccurate results and hinder the determination of the endpoint.
A Symphony of Measurements
The combination of the graduated cylinder and burette provides a harmonious symphony of measurements. The graduated cylinder caters to larger volumes, while the burette’s finesse handles smaller volumes with unmatched accuracy. Together, they form an indispensable duo in the pursuit of robust and reliable titration results.
Epilogue
Precise volume measurements in titration are the cornerstone of accurate and meaningful results. They pave the way for unraveling the complexities of acid-base reactions and other chemical interactions. By wielding the graduated cylinder and burette with utmost precision, chemists embark on a journey of discovery, unlocking the secrets hidden within chemical solutions.
Titration Essentials: A Comprehensive Guide
Unveiling the intricacies of titration is like embarking on a scientific adventure. It’s a technique that allows us to unravel the mysteries of chemical solutions by precisely measuring and reacting them. In this comprehensive guide, we’ll delve into the world of titration, from its basic concepts to the essential tools used in this fascinating process.
Understanding Titration
At the heart of titration lies the equivalence point, a critical moment when two solutions perfectly neutralize each other. This concept is central to titration, and we’ll explore how to recognize it through visual cues like changes in pH and color.
Role of Indicators
Indicators are the unsung heroes of titration. These special chemicals, like phenolphthalein, possess the ability to signal the completion of a reaction by changing color. Their clever use ensures accurate determination of the equivalence point.
Graduated Cylinder: Measuring with Precision
Measuring volume precisely is crucial in titration. Graduated cylinders are our trusty tools for this task. They allow us to accurately dispense known volumes of liquids, setting the stage for successful titrations.
Burette: Dispensing with Finesse
The burette, a more sophisticated measuring instrument, takes precision to new heights. Its precise and calibrated design allows us to dispense precise volumes of liquid, ensuring reproducible results in our titrations.
Titration, with its interplay of chemistry and precision, is a cornerstone of scientific experimentation. By understanding the concepts of titration and mastering the use of indicators, graduated cylinders, and burettes, we can unlock the secrets of chemical reactions and make informed decisions in our research and beyond.
Calibration and maintenance of burettes for accurate liquid dispensing
II. The Equivalence Point
Determining the equivalence point, the critical juncture where reactants are completely neutralized, is pivotal in titration. This point marks the moment of stoichiometric balance, when the moles of acid and base are in exact proportion. Recognizing the equivalence point is crucial for accurate analysis.
III. Neutralization Reaction
Titration relies on the neutralization reaction between an acid and a base. This reaction forms a neutral salt and water, releasing heat in the process. The equivalence point signifies the complete neutralization of the acid and base, resulting in a solution with a neutral pH.
IV. pH and Equivalence Point
Understanding pH is essential for determining the equivalence point. pH measures the acidity or alkalinity of a solution on a scale from 0 to 14. At the equivalence point, the pH of the solution is 7, indicating neutrality. However, the exact pH at the equivalence point varies depending on the strengths of the acid and base used.
V. Phenolphthalein as an Indicator
Indicators are substances that signal the completion of a reaction by changing color. Phenolphthalein is a common indicator used in titrations. It remains colorless in acidic solutions and turns a vibrant pink when the solution becomes basic, indicating the equivalence point.
VI. Role of Indicators in Titration
Indicators play a crucial role in titration by providing a visual cue for the completion of the reaction. Different indicators are sensitive to specific pH ranges, allowing them to be used in a variety of titrations. The appropriate indicator must be selected based on the expected pH value at the equivalence point.
VII. Graduated Cylinder: Measuring Volume
Measuring precise volumes is essential in titration. Graduated cylinders are commonly used to measure the volume of the analyte solution. It is important to choose a graduated cylinder with the appropriate range and graduations for the specific volume required.
VIII. Burette: Dispensing Precise Volume
Burettes are specialized glassware used to deliver precise volumes of titrant (the solution of known concentration). They are calibrated with extreme accuracy, allowing for the controlled addition of titrant during the titration process. Proper calibration and maintenance of the burette are crucial to ensure accurate liquid dispensing.
Mastering the Art of Titration: Techniques for Precise Volume Delivery with Burettes
In the realm of chemistry, titration stands as a crucial technique for determining the concentration of an unknown solution. Burettes, precision instruments used in this process, play a pivotal role in delivering precise volumes of solutions, ensuring accurate results.
As you embark on the titration journey, consider these techniques to master the art of precise volume delivery with burettes:
1. Calibrate Your Burette with Utmost Accuracy
Before each titration, calibrate your burette to ensure its precision. Use distilled water to rinse the burette thoroughly, then fill it to the zero mark. Slowly drain the water until it reaches the calibration mark and record the exact volume. Any deviation from the expected volume indicates the need for further calibration.
2. Measure and Dispense with Precision
When measuring a solution into the burette, hold it at eye level to ensure accurate reading. Meniscus, the lower curve of the liquid level, should be aligned with the desired graduation mark.
To dispense the solution precisely, slowly open the burette stopcock while gentling tapping the side of the burette to remove any trapped air bubbles. Maintain a steady flow rate and observe the meniscus carefully as it approaches the desired volume.
3. Optimize Liquid Flow
For viscous liquids, such as concentrated acids or bases, pre-wet the burette with a small amount of the solution before filling it. This helps to reduce the tendency of the liquid to adhere to the walls, ensuring accurate delivery.
4. Practice Makes Perfect
Regular practice is key to mastering the techniques of titration. Perform multiple titrations to familiarize yourself with the equipment and hone your skills. This will increase your confidence and precision in delivering precise volumes.
By following these techniques, you will elevate your titration skills, enabling you to achieve accurate and reliable results in your chemical analyses. Remember, precision is the cornerstone of successful titration, and burettes are your indispensable tools in this endeavor.