Determining Mass Percent Of Hydrogen In Water: A Guide To Composition And Calculations
The mass percent of hydrogen in water is a quantitative measure that expresses the amount of hydrogen present in a given mass of water. It involves understanding the concept of mass percent, the significance of hydrogen in water’s composition, and the role of molecular weight, molar mass, and the mole in determining the mass of substances. By understanding these interconnected concepts, we can calculate the mass percent of hydrogen in water, a crucial parameter in fields such as chemistry, physics, and materials science.
Understanding Mass Percent: A Quantitative Measure for Chemical Composition
In the realm of chemistry, understanding the composition of substances is crucial. Mass percent, a quantitative measure, plays a vital role in determining the relative amount of elements or compounds present within a given sample.
Mass percent is a useful tool for chemists because it expresses the mass of a specific component as a percentage of the total mass of the mixture. This value is often used to compare the composition of different samples, identify unknown substances, and determine the purity of chemicals.
To define mass percent mathematically, it can be expressed as:
Mass percent = (Mass of Component / Total Mass of Mixture) x 100%
For instance, if a sample of water weighs 100 grams and contains 2 grams of hydrogen, the mass percent of hydrogen in the water would be:
Mass percent of hydrogen = (2 g / 100 g) x 100% = 2%
It’s important to note that mass percent is closely related to other quantitative measures, such as percentage and molecular weight. Percentage represents a fraction of a whole expressed as a hundredth, while molecular weight refers to the sum of the atomic weights of all atoms in a molecule.
By understanding the interrelationship between these concepts, chemists can accurately determine the composition of substances and gain valuable insights into their chemical properties and behavior.
Hydrogen’s Role in Water’s Composition
Water, the elixir of life, is a molecule composed of hydrogen and oxygen. Hydrogen, the lightest element, plays a crucial role in determining the unique properties of water.
Hydrogen atoms are tiny and highly reactive, forming covalent bonds with adjacent atoms. In water, each hydrogen atom is covalently bonded to an oxygen atom. These strong bonds create a stable molecular structure.
Hydrogen’s small size allows it to form hydrogen bonds with neighboring water molecules. These hydrogen bonds are weaker than covalent bonds but play a significant role in water’s_ cohesive properties. They attract water molecules to each other, increasing water’s density and surface tension.
Additionally, hydrogen’s low electronegativity makes it slightly positive. This slight polarity contributes to water’s solvating ability. Polar molecules dissolve in water because the positive end of the polar molecule is attracted to the negative end of the water molecule, while the negative end of the polar molecule is attracted to the positive end of the water molecule.
In summary, hydrogen’s unique properties – small size, low electronegativity, and high reactivity – enable it to form strong covalent bonds with oxygen atoms and hydrogen bonds with neighboring water molecules. These interactions determine the molecular structure and properties of water, making it essential for life on Earth.
Molecular Weight: Determining the Mass of Molecules
In the realm of chemistry, our understanding of the microscopic world relies heavily on quantifying the composition of substances. Molecular weight is a crucial concept that helps us unravel the mass of molecules, the fundamental building blocks of matter.
Imagine you’re building a house. To determine the total weight of the structure, you’d add up the individual weights of each brick, plank, and piece of metal used. Similarly, molecular weight represents the sum total of the atomic weights of all the atoms in a molecule. Each atom has a specific weight, and by adding up these weights, we can determine the overall mass of the molecule.
This concept is particularly relevant for complex molecules, such as those in living organisms or advanced materials. By knowing the molecular weight, we can estimate the total mass of a large number of molecules, making it easier to work with and analyze large quantities of substances.
Furthermore, molecular weight has a direct relationship with molar mass. Molar mass refers to the mass of one mole of a substance. One mole is a specific quantity of substance, equal to 6.022 × 1023 entities (atoms, molecules, or ions). The molecular weight of a substance is numerically equal to its molar mass, expressed in grams per mole (g/mol).
Understanding the concept of molecular weight is essential for various scientific fields, including chemistry, biochemistry, and materials science. It enables us to analyze the composition of substances, predict their properties, and design new materials with desired characteristics.
Molar Mass: Unveiling the Mass of a Substance
- Define molar mass as the mass of one mole of a substance.
- Connect molar mass to molecular weight and the mole concept.
Molar Mass: Unveiling the Mass of a Substance
In the realm of chemistry, understanding the mass of a substance is paramount. To delve into this fascinating concept, we introduce the molar mass, a fundamental unit that allows us to precisely measure and compare the masses of different substances.
The molar mass of a substance is defined as the mass of one mole of that substance. A mole represents a specific quantity, akin to a dozen eggs or a gross of pencils, but in the chemical world, it refers to an astronomical number of particles. Exactly 6.02214076 x 10^23 atoms, molecules, ions, or other particles constitute one mole.
Molar mass bears an intrinsic connection to molecular weight, a term familiar to many. Molecular weight represents the sum of the atomic weights of all atoms within a molecule. While molecular weight measures the mass of an individual molecule, molar mass quantifies the mass of a vast collection of molecules, one mole’s worth.
To further enhance our understanding, let’s ponder the significance of the mole concept. The mole concept provides a bridge between the microscopic world of atoms and molecules and the macroscopic world we experience. By comprehending the molar mass of a substance, we can effortlessly calculate the mass of a specific number of particles or determine the number of particles present in a given mass. In essence, molar mass acts as a universal translator, enabling us to seamlessly convert between the two realms.
The Mole: A Crucial Unit of Measure in Chemistry
In the realm of chemistry, understanding the mole is essential. It’s the unit of measurement for the amount of substance, providing a precise way to quantify the constituents of matter.
The mole has an intriguing history. It emerged as a concept in the early 19th century when chemists recognized the need for a standardized unit to express the relative quantities of reactants and products in chemical reactions. The mole was formally defined in 1967 as the amount of substance that contains the same number of elementary entities as there are atoms in precisely 0.012 kilograms of carbon-12.
The concept of molar mass is closely intertwined with the mole. Molar mass represents the mass of one mole of a substance. It’s essentially the molecular weight of a substance expressed in grams per mole. Through molar mass, we can determine the mass of a specific number of moles of a substance.
The relationship between the mole, molar mass, and Avogadro’s number is fundamental. Avogadro’s number (6.022 × 10^23) represents the number of elementary entities (atoms, molecules, ions, or electrons) present in one mole of a substance. Understanding these connections enables chemists to convert between the mass, moles, and number of elementary entities of a substance.
In practice, the mole plays a crucial role in stoichiometry, the study of the quantitative relationships between reactants and products in chemical reactions. It allows chemists to determine the precise amounts of reactants required and products formed in a reaction. Moreover, the mole concept is indispensable in analytical chemistry, where it’s used to express the concentrations of solutions and determine the amounts of substances present in samples.
In summary, the mole is a fundamental unit of measurement in chemistry. It quantifies the amount of substance and establishes a precise link between the mass, moles, and number of elementary entities of a substance. Understanding the mole and its relationships empowers chemists to perform accurate calculations, analyze chemical reactions, and determine the compositions of chemical substances.
Understanding Percentage: A Tool for Quantifying
In the realm of science and everyday life, we often encounter the term “percentage.” It’s a concept that helps us quantify, or measure, the relative amount of something in a given context.
Mathematically, a percentage is a fraction expressed as a hundredth. In other words, it represents a part of a whole divided by 100. For instance, 50% means half or 50 out of 100.
The versatility of percentage lies in its ability to represent various quantities, from the success rate of a project to the purity of a chemical substance. In science, mass percent is a common way to express the concentration of a solute in a solution.
Mass percent = (Mass of solute / Mass of solution) x 100%
This formula allows us to determine the amount of solute present in a solution based on its mass. For example, a 10% mass percent solution of salt means that for every 100 grams of solution, 10 grams are salt.
Percentage also plays a crucial role in fractional percentages. For instance, 1/4 can be expressed as 25% (1/4 x 100%). This conversion allows us to compare fractions and percentages more efficiently.
Overall, percentage is a powerful tool that helps us quantify and compare parts of a whole. By understanding its definition and applications, we can navigate various scientific and everyday scenarios with ease.
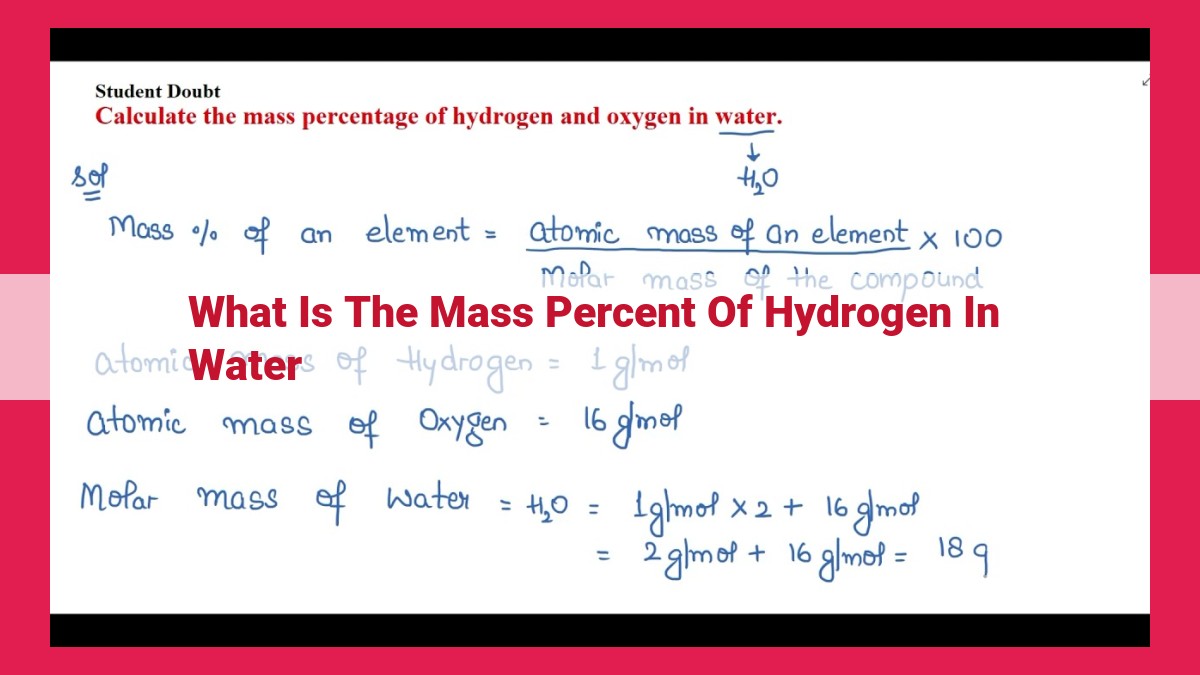
Determining Mass Percent of Hydrogen in Water: A Practical Example
In our exploration of the composition of water, we come to the pivotal concept of mass percent. Mass percent quantifies the relative amount of a specific element or compound in a mixture, providing valuable insights into its chemical makeup.
To calculate the mass percent of hydrogen in water, we embark on a multifaceted journey that intertwines the fundamental concepts of molecular weight, molar mass, and the enigmatic mole.
- Step 1: Unveiling Molecular Weight
The molecular weight of water, H2O, is the sum of the atomic weights of its constituent atoms: 2 hydrogen atoms with an atomic weight of approximately 1 gram each, and 1 oxygen atom with an atomic weight of approximately 16 grams. This yields a molecular weight of (2 x 1) + 16 = 18 grams/mole.
- Step 2: Establishing Molar Mass
The molar mass of water is the mass of one mole of water, which is equal to its molecular weight, 18 grams/mole. Molar mass provides a convenient way to relate the mass of a substance to its amount.
- Step 3: Introducing the Mole
The mole is a fundamental unit of measurement in chemistry, representing a specific quantity (approximately 6.022 x 10^23) of particles. In our case, the particles are water molecules.
- Step 4: Calculating Mass Percent
Now, we can determine the mass percent of hydrogen in water:
- Mass of hydrogen in one mole of water = (2 x 1 gram) = 2 grams
- Mass percent of hydrogen = (Mass of hydrogen / Molecular weight of water) x 100%
- Mass percent of hydrogen = (2 grams / 18 grams/mole) x 100%
- Mass percent of hydrogen = 11.11%
This result reveals that hydrogen constitutes 11.11% of the mass of water, a substantial proportion that underscores its significance in the molecular structure and properties of this ubiquitous compound.