Determining Mass In Grams: A Comprehensive Guide For Accurate Measurement
How to Find Grams:
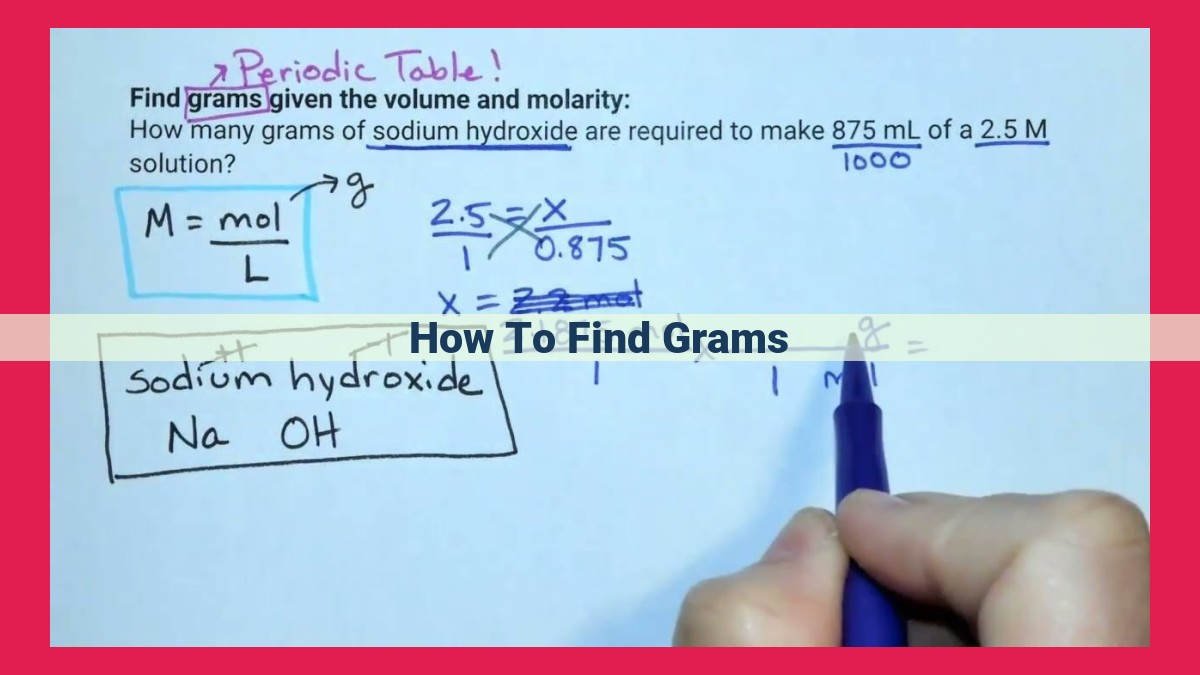
To determine the mass in grams, a multi-step process is required. First, establish the substance’s chemical formula, which provides its molar mass. Next, multiply the molar mass by the quantity in moles to obtain the total mass. If the substance’s volume is known, multiply the volume by its density to calculate its mass. Alternatively, employ equipment like balances to directly measure the mass in grams. These methods provide precise mass determination, enabling accurate calculations and experiments.
Understanding the Importance of Mass
Mass, an intrinsic property of matter, plays a pivotal role in our scientific understanding and everyday lives. Mass quantifies the amount of matter in an object, providing crucial insights into its physical and chemical properties.
In science, mass is the cornerstone of Newtonian mechanics. It governs the motion of objects, influencing their acceleration and gravitational force. In chemistry, mass is essential for stoichiometry, determining the exact quantities of reactants and products in chemical reactions. Understanding mass empowers us to predict the outcomes of countless chemical processes.
Beyond the scientific realm, mass impacts our daily lives. From determining the weight of groceries to calibrating the tires of our cars, the concept of mass permeates various aspects of our existence. By accurately measuring mass, we can ensure the safe and efficient functioning of countless devices and systems, from bathroom scales to spacecraft.
The Mole: A Bridge to Understanding Matter’s Foundation
In the intricate realm of science, where countless atoms dance and collide, the mole stands as a pivotal concept, offering us a deeper understanding of the fundamental building blocks of our universe. Picture it: a specific amount, like a dozen eggs or a ream of paper, but for the tiniest particles known to man.
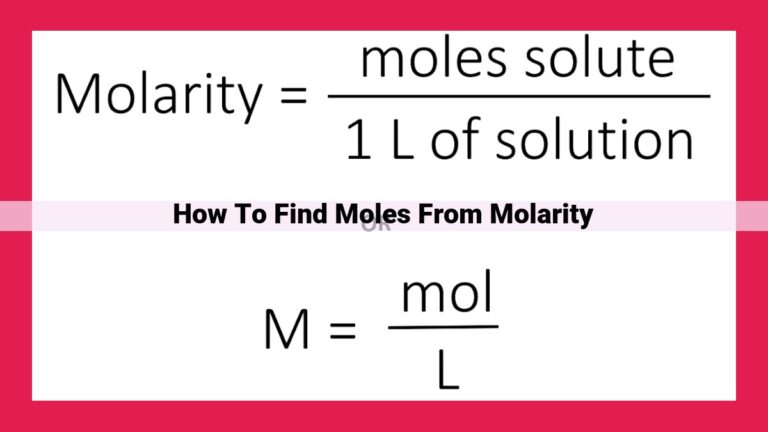
The mole, denoted by the symbol “mol,” is an SI unit specifically devised to quantify atoms, molecules, and ions. It allows us to comprehend the immense numbers of these particles that can exist in a given sample, bridging the gap between microscopic and macroscopic scales.
The core of the mole concept lies in Avogadro’s number (6.022 × 1023), a numerical constant that represents the staggering number of particles present in one mole of any substance. This fundamental constant serves as a conversion factor between the microscopic world of atoms and the tangible quantities we measure in the laboratory.
Furthermore, understanding the atomic mass of an element is crucial. The atomic mass, a dimensionless quantity, signifies the average mass of an element’s naturally occurring isotopes, weighted according to their relative abundances. It gives us a glimpse into the composition of matter itself.
By knowing the atomic mass and Avogadro’s number, we can determine the molar mass of a substance, a value that represents the mass in grams of one mole of that substance. This knowledge empowers us to connect the mass of a substance to the number of particles it contains, a pivotal step in unraveling the inner workings of chemical reactions.
Calculating Molar Mass: The Key to Understanding Substance Composition
In chemistry, understanding the makeup of substances is crucial. One key factor in this is molar mass, the mass of one mole of a compound. A mole is a unit of measurement equal to Avogadro’s number (6.022 × 10^23) of particles (atoms, molecules, or ions).
Molecular weight and formula mass are terms often used interchangeably with molar mass. Molecular weight refers to the mass of one molecule, while formula mass refers to the mass of one unit of a compound. These values are determined by adding the atomic masses of all the elements present in the compound. Atomic mass is the weighted average of the masses of all the isotopes of an element.
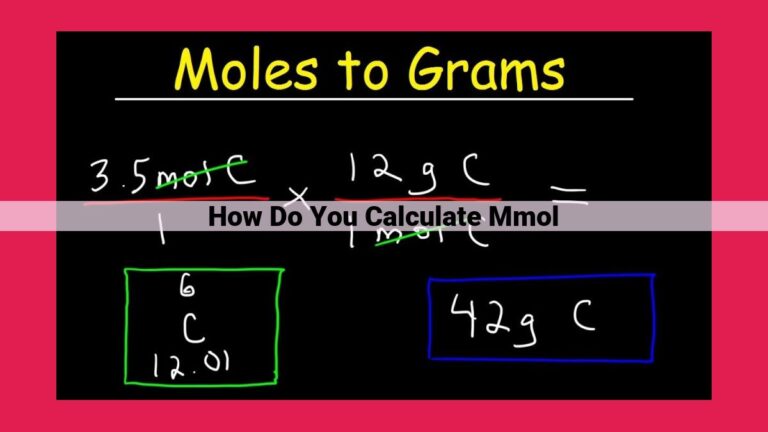
Calculating molar mass is simple. Let’s say we want to find the molar mass of glucose (C6H12O6). First, we multiply the atomic mass of each element by its number of atoms in the molecule:
- Carbon (C): 6 atoms × 12.011 amu = 72.066 amu
- Hydrogen (H): 12 atoms × 1.008 amu = 12.096 amu
- Oxygen (O): 6 atoms × 16.000 amu = 96.000 amu
Adding these values together, we get the molar mass of glucose:
Molar mass of glucose = 72.066 amu + 12.096 amu + 96.000 amu = 180.162 amu
Knowing the molar mass of a compound allows us to convert between mass and number of particles. For example, if we know that the molar mass of sodium chloride (NaCl) is 58.44 g/mol, we can calculate the number of moles of sodium chloride in a 100-gram sample:
Number of moles = Mass / Molar mass
= 100 g / 58.44 g/mol
= 1.71 moles
Calculating molar mass is essential for quantitative analysis and understanding the chemical composition of substances. By using the molecular structure and atomic masses, we can accurately determine the mass of one mole of a compound, unlocking a wealth of information about its composition and properties.
Mass Measurement Techniques
Defining Mass: The Essence of Matter
Mass is a fundamental physical property of matter, quantifying the amount of matter present in an object. It is distinct from weight, which measures the gravitational force acting on an object. Mass remains constant regardless of its location or the presence of gravitational forces.
Inertia: The Resistance to Change
The concept of inertia is closely tied to mass. Inertia refers to an object’s resistance to any change in its motion. An object with greater mass possesses greater inertia, requiring more force to accelerate or decelerate it. This property is often observed in everyday life, from pushing a heavy object to braking a vehicle.
Gravitational Force: A Universal Attraction
Gravitational force is another key concept related to mass. It represents the attractive force between two objects due to their masses. The greater the mass of an object, the stronger its gravitational pull. This force is responsible for phenomena such as the motion of planets around the sun and the Earth’s gravitational pull on us. Understanding mass and its measurement techniques is crucial for comprehending countless scientific and real-world applications.
Measuring Volume: A Dive into the World of Capacity and Displacement
Volume, the measure of space occupied by a substance, is a fundamental concept with wide-ranging applications. From determining the amount of liquid in a bottle to calculating the buoyancy of a ship, understanding volume is essential.
In this exploration, we will delve into the concept of volume, exploring its significance and the techniques used to measure it. We will define key terms like capacity and displacement and discuss the various units of measurement used to quantify volume. Additionally, we will explore methods for determining the volume of liquids and gases, ensuring a comprehensive understanding of this fascinating aspect of science.
Capacity: The Potential to Hold
Capacity refers to the maximum amount of substance that a container can hold. Whether it’s a measuring cup, a bottle, or even a bathtub, the capacity of a container determines its ability to store volume. Often expressed in units of liters (L) or milliliters (mL), capacity is a measure of the potential space a substance could occupy.
Displacement: The Volume an Object Occupies
Displacement, on the other hand, measures the actual volume occupied by an object when it is submerged in a fluid. When an object is placed in a liquid, it displaces an equal volume of that liquid. This displaced liquid provides a direct indicator of the object’s volume. Archimedes’ principle, a cornerstone of buoyancy, is based on this concept of displacement.
Measuring Volume: Methods and Units
The precise measurement of volume is crucial in various scientific and practical applications. Several techniques are employed to determine the volume of liquids and gases, including:
- Graduated cylinders and beakers provide accurate and convenient measurements of liquids, marked with volumetric scales.
- For smaller volumes, pipettes and burettes are used for precise liquid dispensing.
- Measuring the mass and density of a liquid can also provide volume estimates.
- Gas syringes and displacement methods are commonly used to measure the volume of gases.
Understanding volume is essential in numerous fields, such as chemistry, physics, and engineering. By exploring the concepts of capacity and displacement, and familiarizing ourselves with the techniques for measuring volume, we gain a deeper appreciation of this fundamental aspect of our physical world.
Calculating Density: Unveiling the Essence of Matter
Density, the intrinsic characteristic of matter, unveils the fundamental relationship between mass and volume. It measures how tightly packed atoms or molecules are within a substance. Understanding density empowers us to determine the compactness, buoyancy, and other critical properties of various materials.
Mass and Volume: A Tale of Two Measures
Mass, an immutable property, quantifies the amount of matter in an object, while volume measures the space it occupies. These concepts are intertwined in the calculation of density. A substance with a larger mass relative to its volume will exhibit a higher density.
Buoyancy and Specific Gravity: Weightless Wonders
Buoyancy, the upward force exerted by a fluid on an immersed object, is directly related to density. Objects with a lower density than the surrounding fluid will float, while objects with a higher density will sink.
Specific gravity, a dimensionless quantity, compares the density of a substance to the density of water. A specific gravity greater than 1 indicates a higher density than water, while a specific gravity less than 1 indicates a lower density than water.
The Formula for Density: A Universal Equation
Density is calculated using the simple formula:
Density = Mass / Volume
where:
– Density is measured in grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³)
– Mass is measured in grams (g) or kilograms (kg)
– Volume is measured in cubic centimeters (cm³) or cubic meters (m³)
By understanding the concepts of mass, volume, buoyancy, and specific gravity, we can harness the power of density to delve into the inner workings of matter and predict its behavior under different conditions.