Manganese: Chemical Properties, Atomic Structure, And Electron Configuration
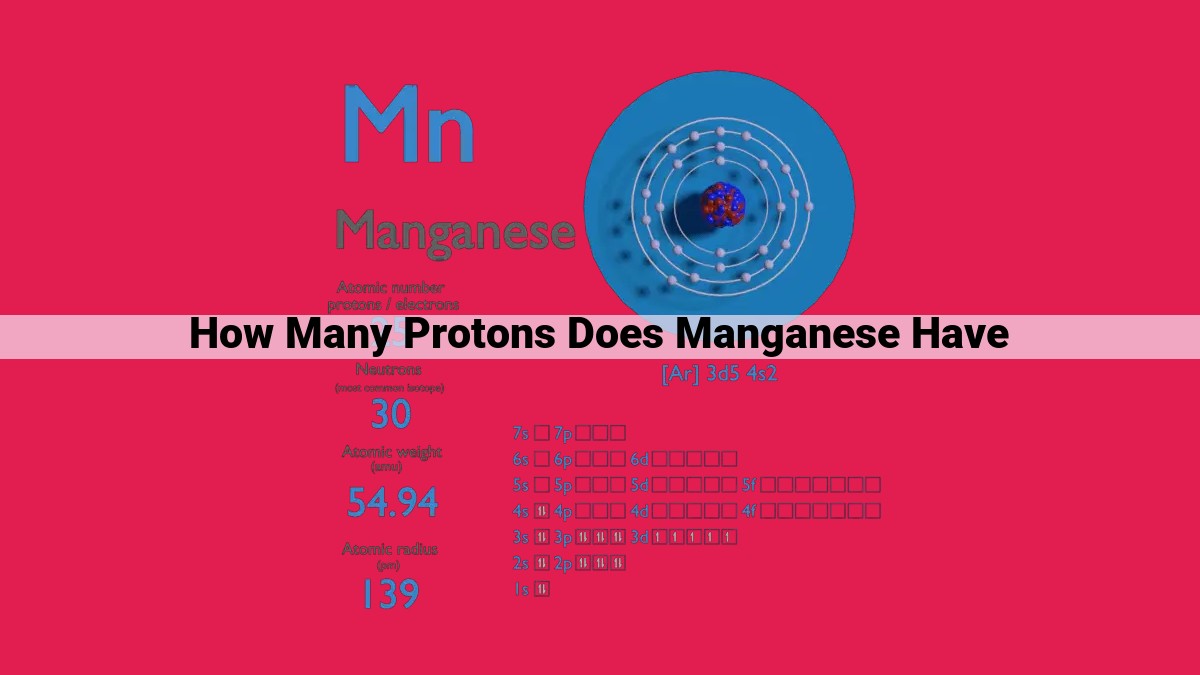
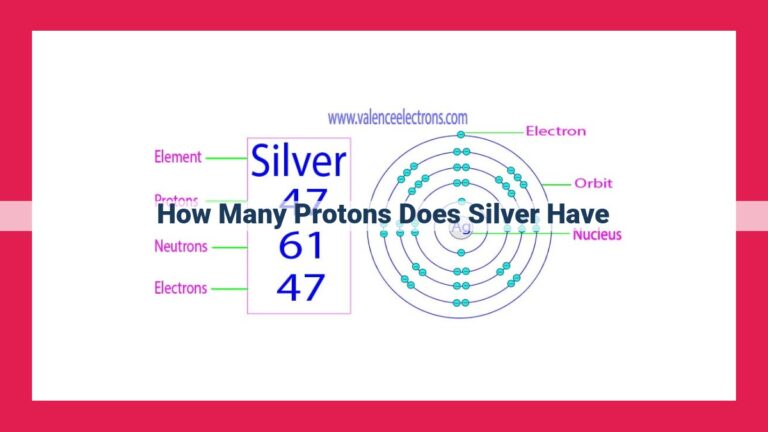
Manganese, denoted by the chemical symbol Mn, has an atomic number of 25. This means that each manganese atom contains 25 protons in its nucleus, which defines its elemental identity. The number of protons in an atom determines its atomic number and distinguishes it from all other elements. Manganese’s atomic structure comprises 25 protons, 30 neutrons, and 25 electrons. Its electron configuration, 1s²2s²2p⁶3s²3p⁶4s²3d⁵, signifies that it has five d-orbital electrons, contributing to its unique chemical reactivity and magnetic properties.
Understanding the Atomic Structure of Manganese: A Journey into the Heart of Matter
Every element in the vast tapestry of the universe is defined by its unique number of protons, neutrons, and electrons. Among these elements, manganese holds an intriguing place, boasting a rich atomic structure and a diverse array of properties.
At the core of every manganese atom lies its nucleus, a dense and positively charged particle. Within this nucleus reside 25 protons, forming the cornerstone of the element’s identity. These protons determine the element’s atomic number, a fundamental characteristic that distinguishes it from all others.
Imagine embarking on an atomic voyage, delving deep into the heart of manganese. As you approach its nucleus, you’ll encounter a bustling hub of activity. The 25 protons, each carrying a single positive charge, whirl around in a furious dance. Their collective presence defines manganese’s unique position on the periodic table.
Unlocking the Secrets of Manganese: The Atomic Number Unveiled
In the vast world of elements, each one holds a unique identity, defined by its atomic number. This number plays a pivotal role in shaping the properties and behavior of an element, and for manganese, this number is 25.
The atomic number of an element represents the number of protons residing in its nucleus, the heart of the atom. Protons carry a positive electrical charge, and their presence dictates the element’s position on the periodic table. Manganese, with 25 protons, is thus located in the 25th position on this table of elements.
This atomic number distinguishes manganese from its neighbors. Every element possesses a unique atomic number, a fingerprint that sets it apart. This individuality is crucial for determining the chemical properties of each element and its ability to react with others.
Understanding the atomic number of manganese is essential for comprehending its nature and behavior. It’s the foundation upon which the element’s atomic structure and electron arrangement are built. With this knowledge, scientists can unravel the intricate details of manganese’s interactions and its contributions to our world.
Manganese Atomic Structure:
- Describe the atomic structure of manganese, including its nucleus and electron arrangement.
- Explain the number of protons, neutrons, and electrons in manganese.
Manganese’s Atomic Structure: A Journey into its Nucleus and Electron Cloud
Manganese, a fascinating element with a remarkable number of 25 protons, occupies a unique position on the periodic table. Its atomic structure, the blueprint of its very existence, reveals a world of intricate details about this enigmatic element.
At the heart of every manganese atom lies a nucleus, a dense core that houses the element’s positive charge. Within this nucleus, 25 protons reside, each contributing a single positive charge. These protons are the defining feature of manganese, giving it an atomic number of 25. The atomic number, a unique identifier for each element, corresponds directly to the number of protons in the nucleus.
Surrounding the nucleus is a cloud of electrons, negatively charged particles that balance the positive charge of the protons. Manganese atoms possess 25 electrons, arranged in a specific configuration that determines the element’s chemical properties. The electrons occupy various energy levels, or shells, around the nucleus.
The inner shell, closest to the nucleus, holds two electrons. The second and third shells accommodate eight and six electrons, respectively. The outermost shell, the valence shell, contains five electrons. This arrangement, known as the electron configuration, is 1s²2s²2p⁶3s²3p⁶4s²3d⁵.
The distribution of electrons in the valence shell significantly influences manganese’s chemical behavior. These valence electrons are responsible for forming bonds with other atoms, determining the element’s reactivity and the compounds it can form.
Comprehending manganese’s atomic structure provides a fundamental understanding of its identity and properties. The number of protons in the nucleus defines its place on the periodic table, while the arrangement of electrons determines its chemical characteristics. This intricate dance of protons and electrons within the manganese atom serves as a testament to the complexity and beauty of the atomic realm.
Manganese Electron Configuration:
- Present the electron configuration of manganese (1s²2s²2p⁶3s²3p⁶4s²3d⁵).
- Explain the distribution of electrons in the electron shells.
- Discuss the implications of the electron configuration for chemical bonding.
Manganese Electron Configuration: A Journey into the Realm of Elements
Delving into the intricate world of elements, we encounter manganese, a fascinating element with a unique atomic structure. Manganese’s electron configuration, represented by the enigmatic string of characters 1s²2s²2p⁶3s²3p⁶4s²3d⁵, holds the key to understanding its chemical identity and behavior.
Each element possesses a distinct atomic number, which corresponds to the number of protons in its nucleus. For manganese, this number is 25, indicating the presence of 25 positively charged protons. The atomic number not only defines an element but also serves as a gateway to its electron configuration.
The electron configuration of manganese reveals the arrangement of its electrons within distinct energy levels or shells. The innermost shell, designated as 1s, accommodates a pair of electrons, followed by the 2s shell with another pair. The third shell, 2p, holds six electrons in three pairs. Moving further out, the 3s shell hosts a second pair of electrons, while the 3p shell houses six electrons in three pairs. Finally, the outermost shell, 4s, contains two electrons, and the 3d shell hosts five electrons.
This distribution of electrons has profound implications for manganese’s chemical bonding capabilities. The outermost electrons, known as valence electrons, play a crucial role in determining an element’s reactivity and bonding behavior. In the case of manganese, it has seven valence electrons, distributed across the 4s and 3d orbitals. These valence electrons are the “social butterflies” of the atomic world, eager to interact with other elements to form chemical bonds.
The unique electron configuration of manganese gives rise to its versatile bonding characteristics. It can exhibit multiple oxidation states, ranging from -3 to +7, reflecting its ability to gain or lose electrons in chemical reactions. This versatility allows manganese to form a wide array of compounds with varying properties, contributing to its diverse applications in various fields, including metallurgy, chemistry, and catalysis.